The Xbiom™ Repository holds all study data managed by Xbiom in a future-proofed, normalized to a simple data model, harmonized to a global terminology set that is mapped to de jure controlled terminology used when exchanging data. The studies include in-vitro and specialty assays from bio-banked bio-samples, in-vivo animal study data, and clinical trial data.
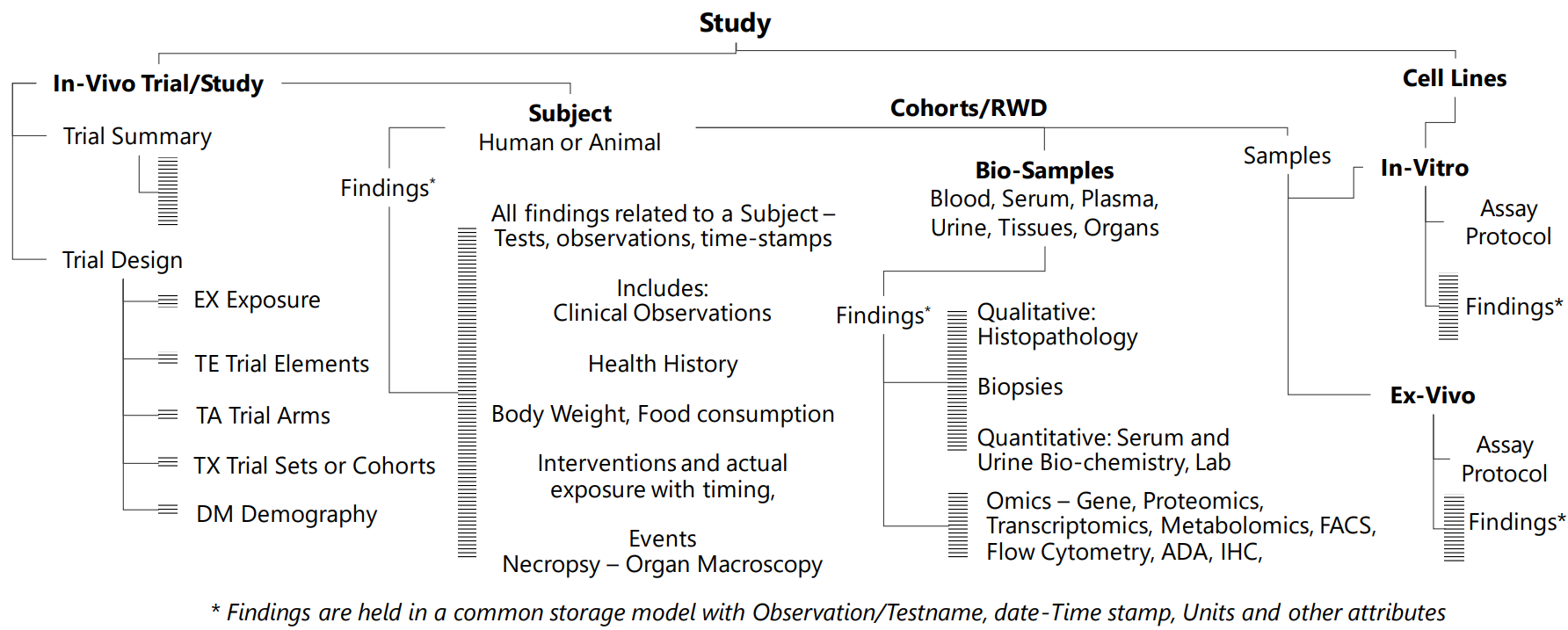
Xbiom’s UDM accommodates the longitudinal representation of as-collected data along with an unlimited number of metadata and derived metadata. These serve various planned and ad hoc access or transformation needs such as standardization for exchange of data to external agencies.
Xbiom indexes this repository to serve search queries. Query masks may cover disparate data domains, they may be hierarchical and recursively applied for evolving repositories.
UDM manages large amounts of specialty biomarker assays from genomics, cell phenotyping, immune-markers and histopathology. It is a valuable research tool especially when the biomarker data longitudinally integrated to the patient’s in-vivo data from clinical trials. Large BioPharma companies also use the UDM to hold all their safety study data from animal studies.
The UDM repository is the keystone of the entire lifecycle of clinical and study data, serving ad hoc research for insights, submission ready standardization using CDISC SDTM, ADaM, and exploratory research leveraging search and analysis across studies from legacy, to ongoing.

