INSIGHTS – Clinical BiomarkerKavan Palangappa2023-07-26T05:53:33+00:00
Clinical Trial, Translational & Biomarker Research
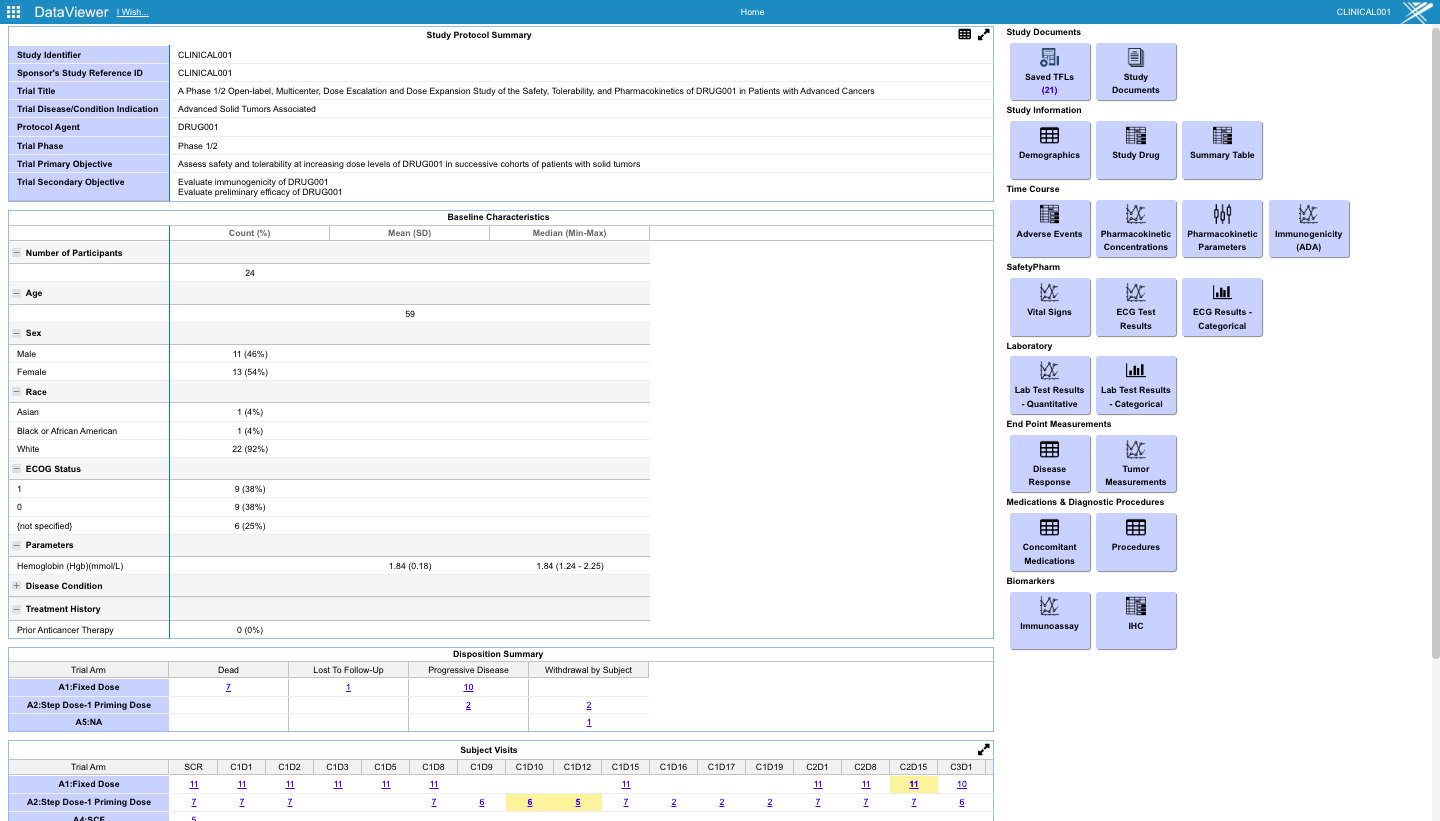
- Monitor ongoing clinical trial EDC data and specialty biomarker assays on patient bio-samples and conduct analysis and visualization in quasi-real time.
- Longitudinal integration of EDC end-points on patients with their biomarker data in near real time.
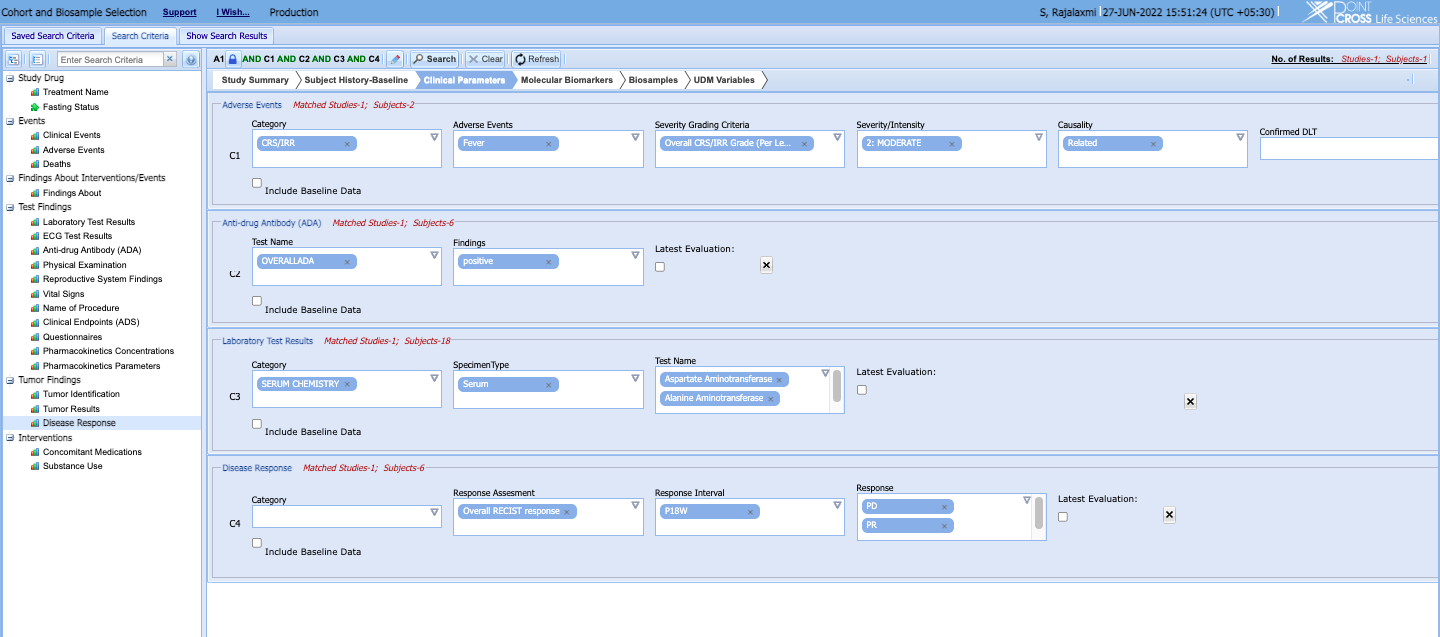
- Search, find and select highly stratified cohorts based on their clinical end-points, biomarker data, health history, concomitant medications and other data.
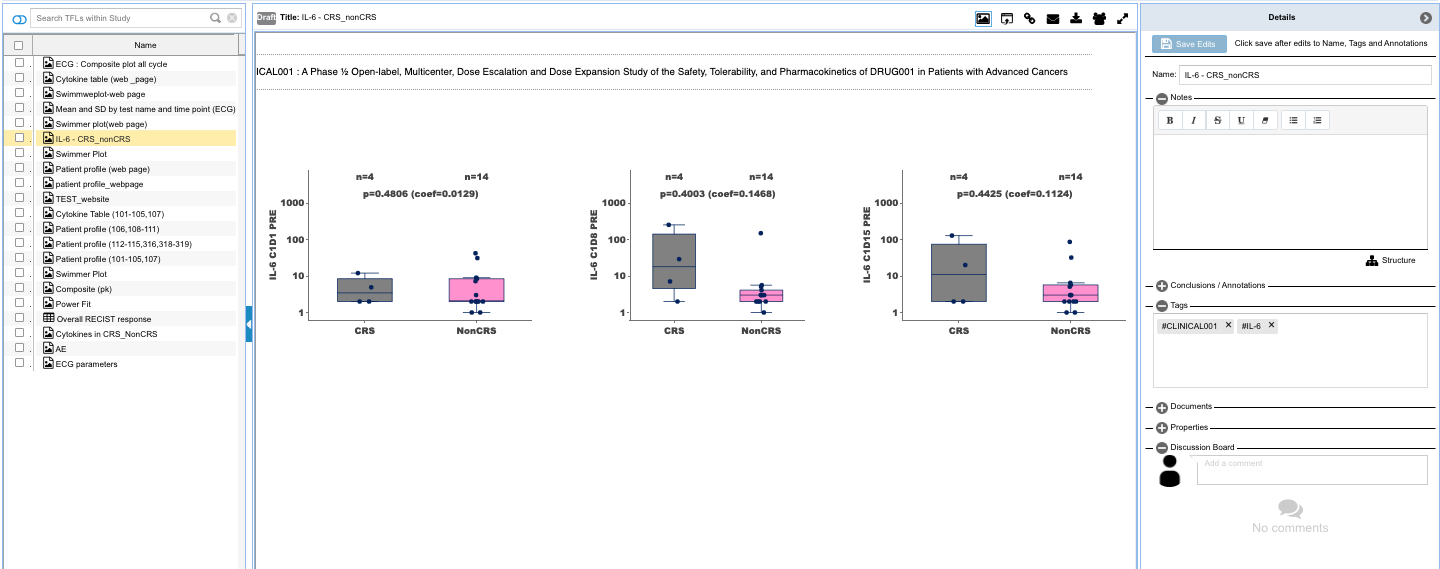
- Compare or cross analyze data from various selected cohorts or trial arms
- Preconfigured monitoring tables and charts accessible from the study dashboard.
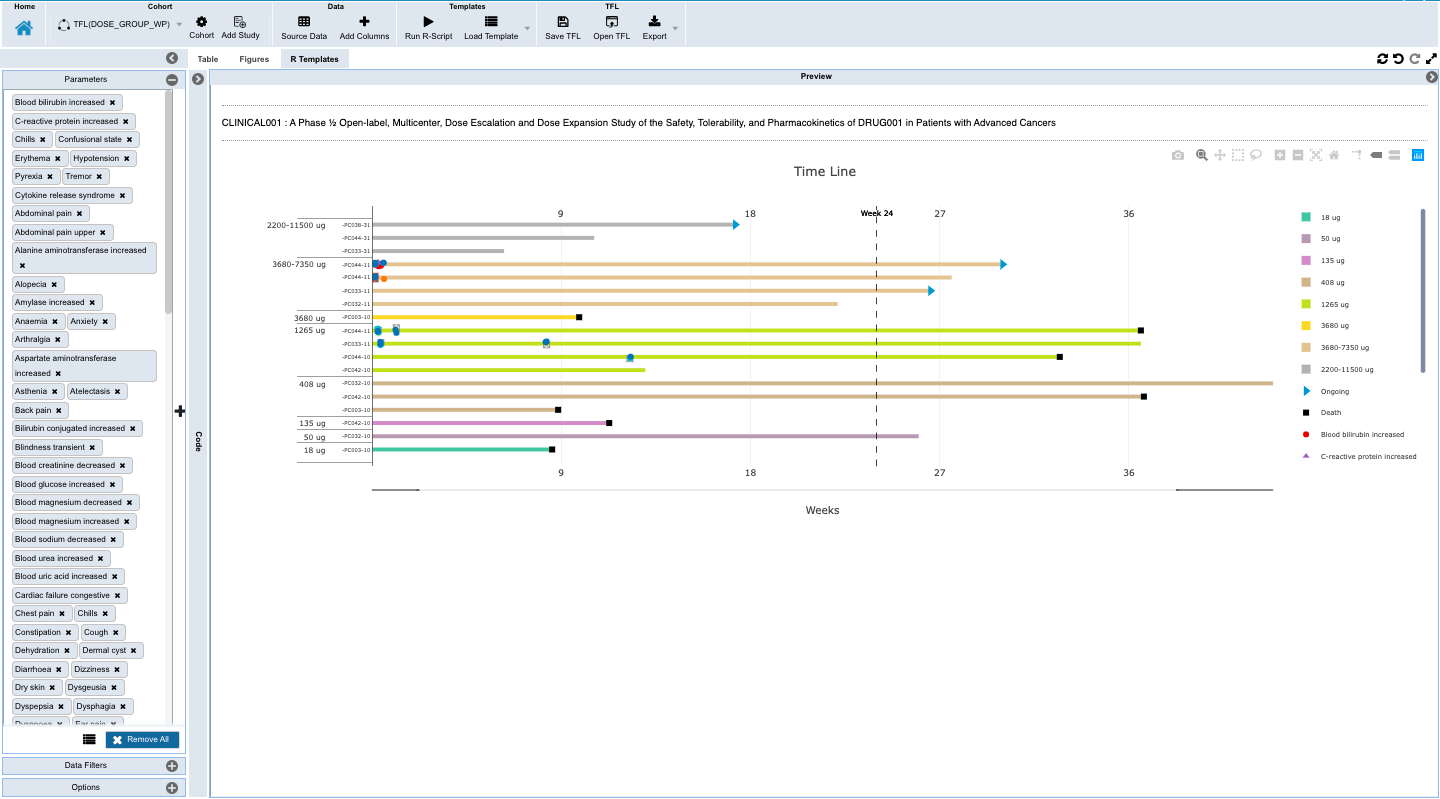
- Overall study timeline view of the ongoing study with events
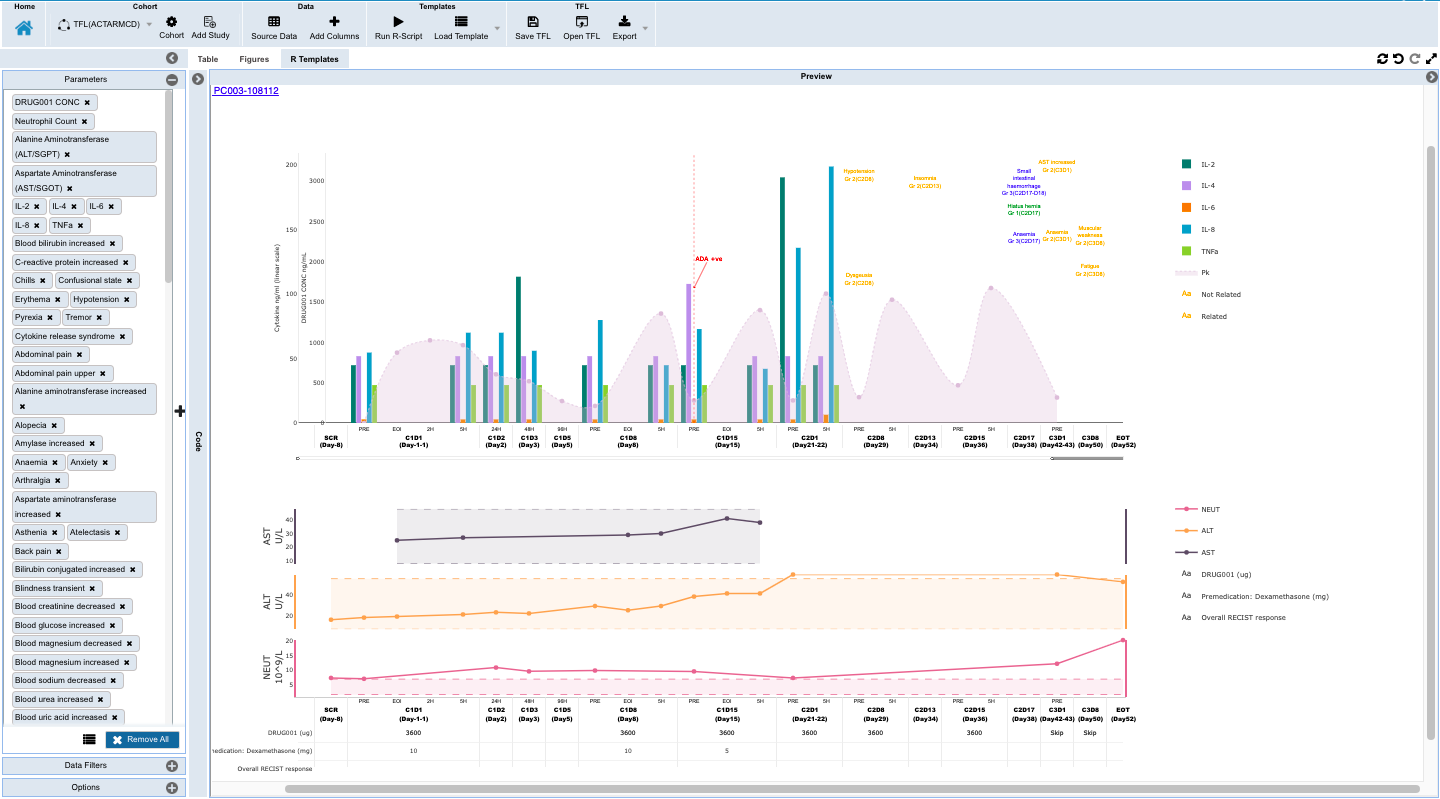
- Monitoring individual patients for safety and efficacy in integrated longitudinal view for dosing, events, lab, PK and biomarker data.
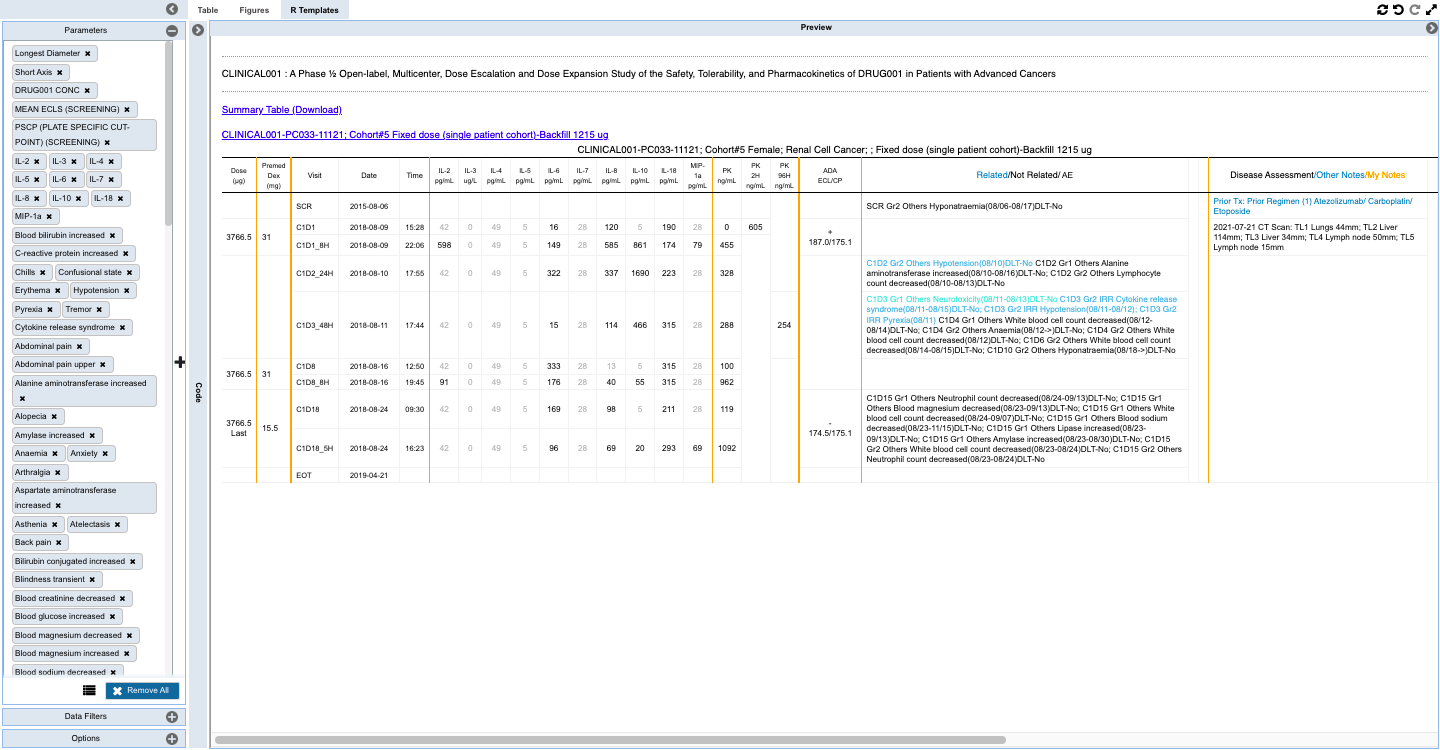
- To scan through the tabulated data for each subject integrated with EDC, PK and Biomarker data along with curated notes
- Stratified search and cohort building
- Customized statistical analysis of the cohort with provision to save as a TFL in your personal workspace or collaborate with team for insight generation