Data Concierge Service
Data Concierge is a white-glove service delivered virtually by an extensive team of data management, data scientists, toxicologists, bio-informatics and biomarker specialists with background and knowledge of data standards (SDTM, ADaM, SEND, and Controlled Terminologies) to help deliver actionable analysis, visualization, and tabulation of study or trial data.
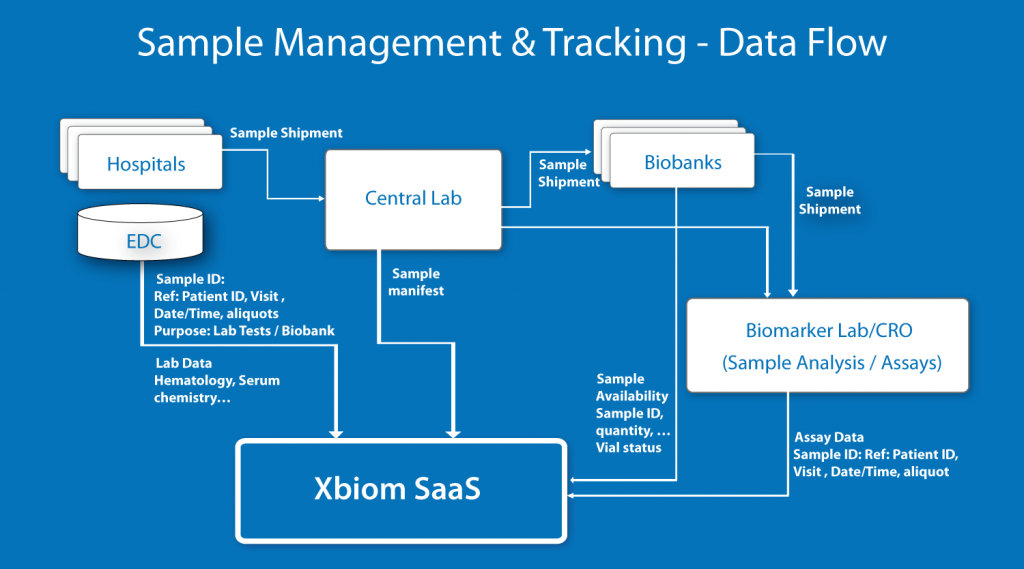
BioTechs choose Xbiom™ to capture, integrate and analyze data from their ongoing nonclinical studies using interim data loads from their CRO LIMS systems or EDCs and biomarker assay stores, and the various bio-analytics and specialty assays labs. This service is powerful for research and safety in nonclinical studies or clinical trials as well as for regulatory preparation and analysis of these studies.
Data Concierge is a powerful solution for BioTechs stretching their operating funds to achieve certain key milestones, or to avoid overhead expenses while focusing on the growth of their key strategic programs.
The service brings speed, agility, business continuity, and rapid delivery of actionable data, tabulations, visualizations, and publishable TFLs and results from the emerging data in the studies or trials.