Faster Report Delivery
Report-SEND Alignment
Cost Reduction per Study
Weeks to SEND
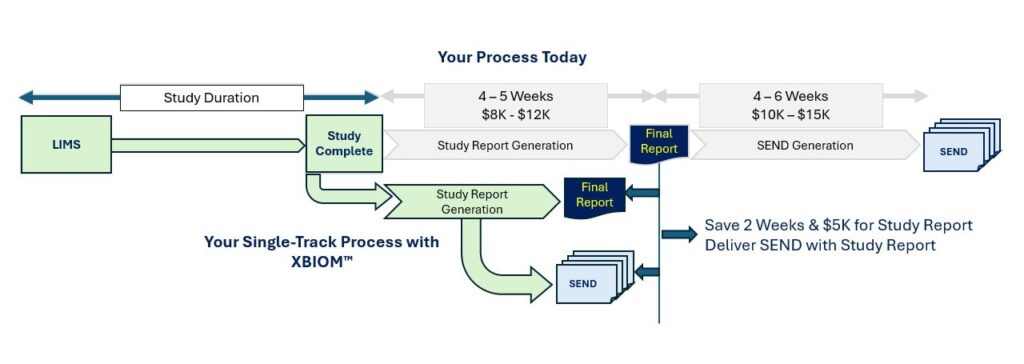
Study Report & SEND timelines take 8+ weeks at traditional CROs
Weeks of Manual Work
Study directors spend hours manually transcribing data from LIMS, histopathology systems, and bioanalytical platforms into Word templates—a time-intensive, error-prone process.
Duplicated Effort, Compounded Delays
SEND teams wait for Study Report completion, then separately wrangle the same source data to create SEND datasets. This duplicated effort adds 5-6 weeks of unnecessary lag time and introduces reconciliation risks.
Quality & Compliance Risk
Manual transcription and dual data entry create discrepancies. Late-stage corrections cascade through both deliverables, triggering costly rework cycles and jeopardizing submission timelines for Sponsors.
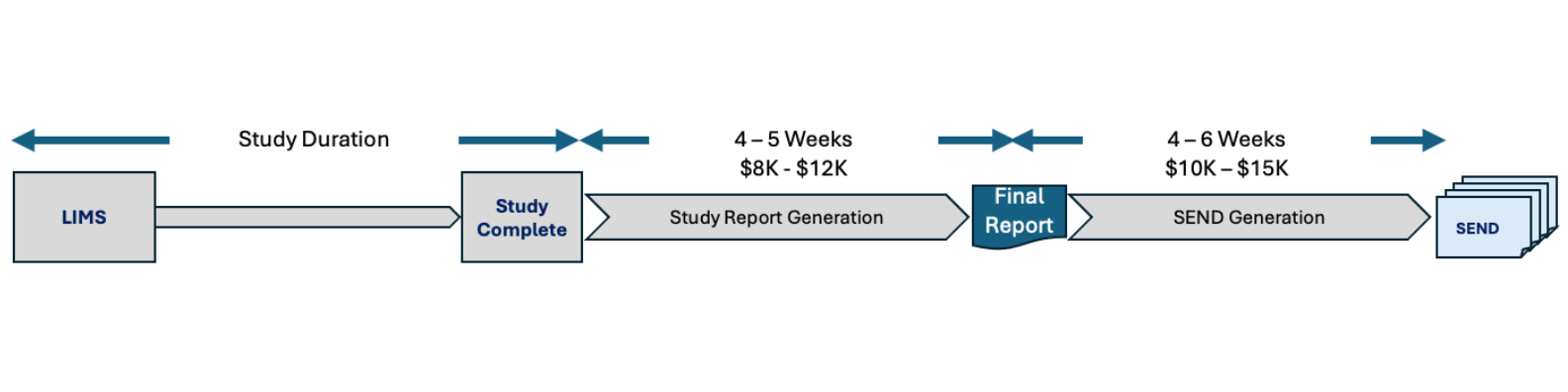
LEGACY PROCESS TAKES 8-10+ WEEKS FOR STUDY REPORT AND SEND GENERATION
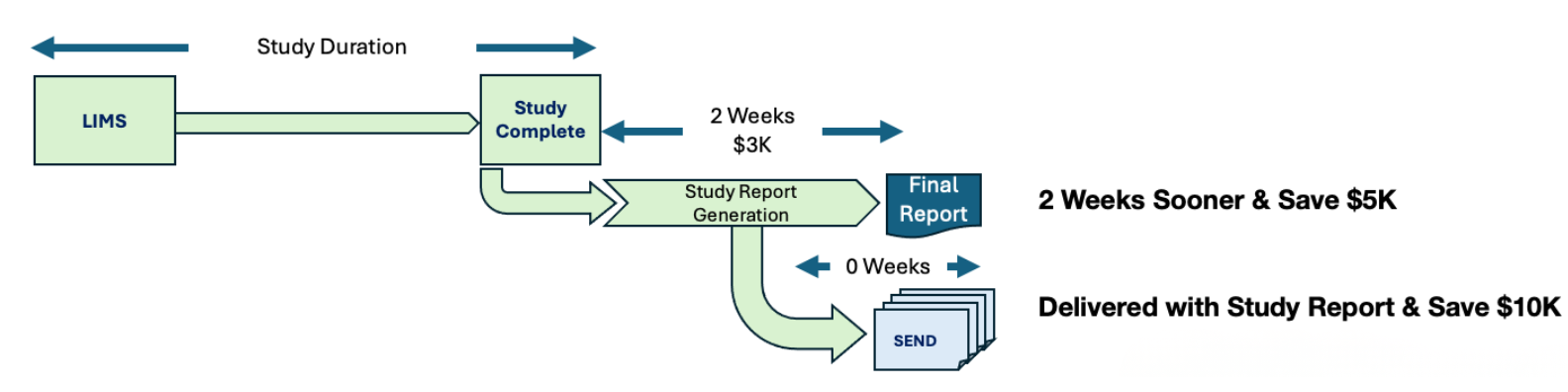
Study Reports Delivered 2 Weeks Faster; SEND Dataset Available at Time of Study Report Generation
-
✓AI-Generated Narratives with Study Director Review
Xbiom™ generates Report Sections based on Study Director and Study Templates, Actual data and records from Study Conduct, and where needed in comparison with Protocol and CoA provided by Sponsor Client. Create coherent, compliant study narratives automatically, following regulatory standards and your organization's templates.
-
✓SEND Generation at the Time of Study Report Finalization
Study reports and SEND datasets generated simultaneously from the same source data, ensuring perfect alignment and minimizing time for reconciliation.
-
✓Full Traceability
Every data point traces back to as-collected source data with complete audit trails. Changes propagate automatically with version management to maintain consistency between Study Report and SEND.
-
✓Smart Quality Checks
Built-in validation checks for regulatory-submission compliant SEND datasets
Transform your CRO's efficiency and competitiveness
Faster Study Report Delivery
Complete study reports that previously took weeks now ready 2 weeks faster. Win more competitive bids with accelerated timelines.
Cost Savings on Average for Each SEND Dataset
Re-capture cost savings to improve study margins.
Consistency & Traceability between Study Report and SEND Dataset - Built In to the Single-Track Process
Shared source-of-truth from as-collected data ensures perfect alignment between study reports and SEND datasets. No manual reconciliation delays.
Weeks to SEND
5-6 weeks faster study deliverable package.


