2+ Weeks
Faster Report Delivery
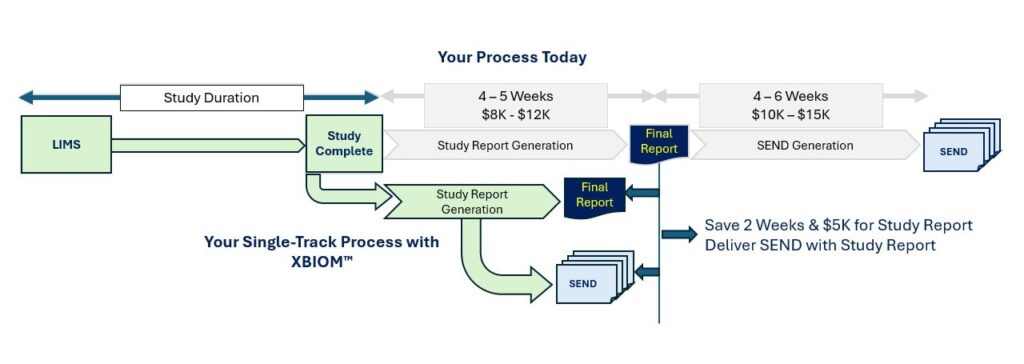
From Data Lock to Sponsor Delivery
100%
Report-SEND Alignment
One Dataset, Two Deliverables: Study Report & SEND
$10k
Cost Reduction per Study
Lower costs. Higher margins. Better outcomes.
0
Weeks to SEND
Instant SEND Dataset on Data Lock
Study Report & SEND timelines take 8+ weeks at traditional CROs
Weeks of Manual Work
Study directors spend hours manually transcribing data from LIMS, histopathology systems, and bioanalytical platforms into Word templates—a time-intensive, error-prone process.
Duplicated Effort, Compounded Delays
SEND teams wait for Study Report completion, then separately wrangle the same source data to create SEND datasets. This duplicated effort adds 5-6 weeks of unnecessary lag time and introduces reconciliation risks.
Quality & Compliance Risk
Manual transcription and dual data entry create discrepancies. Late-stage corrections cascade through both deliverables, triggering costly rework cycles and jeopardizing submission timelines for Sponsors.
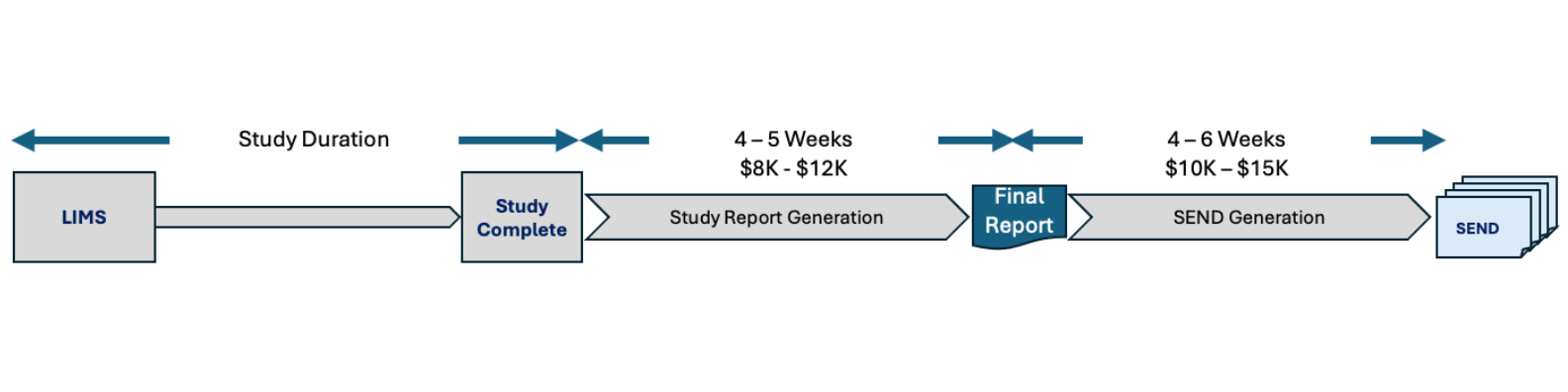
Legacy process takes 8-10+ weeks for study report and SEND generation
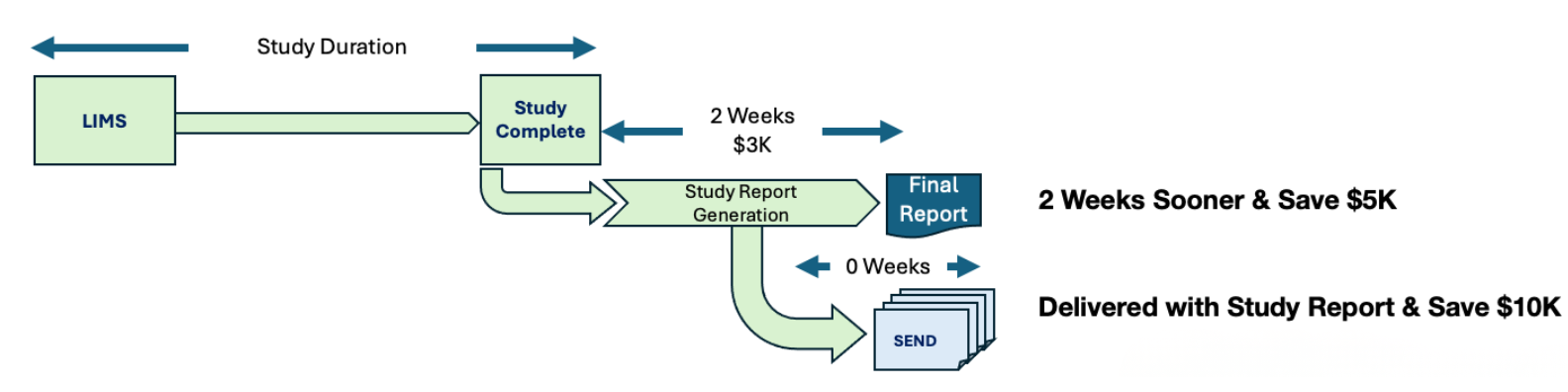
Study Reports Delivered 2 Weeks Faster; SEND Dataset Available at Time of Study Report Generation
LIMS • Histopath • Bioanalytical
shaped in the Study Director's style and report structure; allows Study Director to focus on interpretation of study data, including Pharmacological & Biologic Reasoning, Assessment of Computed Signals
version controlled Study Report and SEND datasets allows review while final, submission ready SEND packages are generated only after Study Data Lock following final approval of Study Report
Study Directors review and approve narrative text, automatically flagged QC findings including master list of deviations from protocol; SEND datasets pass validation and QC checks using Xbiom's facilities including eDataValidator
Xbiom™ generates Report Sections based on Study Director and Study Templates, Actual data and records from Study Conduct, and where needed in comparison with Protocol and CoA provided by Sponsor Client. Create coherent, compliant study narratives automatically, following regulatory standards and your organization's templates.
Study reports and SEND datasets generated simultaneously from the same source data, ensuring perfect alignment and minimizing time for reconciliation.
Every data point traces back to as-collected source data with complete audit trails. Changes propagate automatically with version management to maintain consistency between Study Report and SEND.
Built-in validation checks for regulatory-submission compliant SEND datasets
Transform your CRO's efficiency and competitiveness
Faster Study Report Delivery
Complete study reports that previously took weeks now ready 2 weeks faster. Win more competitive bids with accelerated timelines.
Cost Savings on Average for Each SEND Dataset
Re-capture cost savings to improve study margins.
Consistency & Traceability between Study Report and SEND Dataset - Built In to the Single-Track Process
Shared source-of-truth from as-collected data ensures perfect alignment between study reports and SEND datasets. No manual reconciliation delays.
Weeks to SEND
5-6 weeks faster study deliverable package.


