Introduction
Regulatory agencies like FDA require all nonclinical study data submitted for IND or NDA submissions to be in CDISC SEND format. The Clinical Data Interchange Standards Consortium (CDISC) has established a set of open data standards for the representation and exchange of standardized study data collected over the course of nonclinical and clinical studies. The CDISC Standard for Exchange of Nonclinical Data (SEND) can be used to represent and exchange nonclinical study data. Sponsors must submit data in FDA Supported formats listed in the FDA Data Standards Catalog, which specifies the use of CDISC standards such as SDTM, SEND, ADaM, Define-XML, and Controlled Terminology.
The goal is to have a consistent format for submitting nonclinical study data to the FDA. The format offers increased data quality, accessibility, and predictability, allowing for more effective examination of nonclinical data. Submitting standardized data is a great help to regulatory reviewers, as it enables them to receive, process, review, and archive submissions more effectively. This, in turn, can increase the likelihood of a successful review at the first attempt, and ultimately speed up the process of getting your drug to market. The FDA has designed tools to work with datasets that conform to CDISC standards
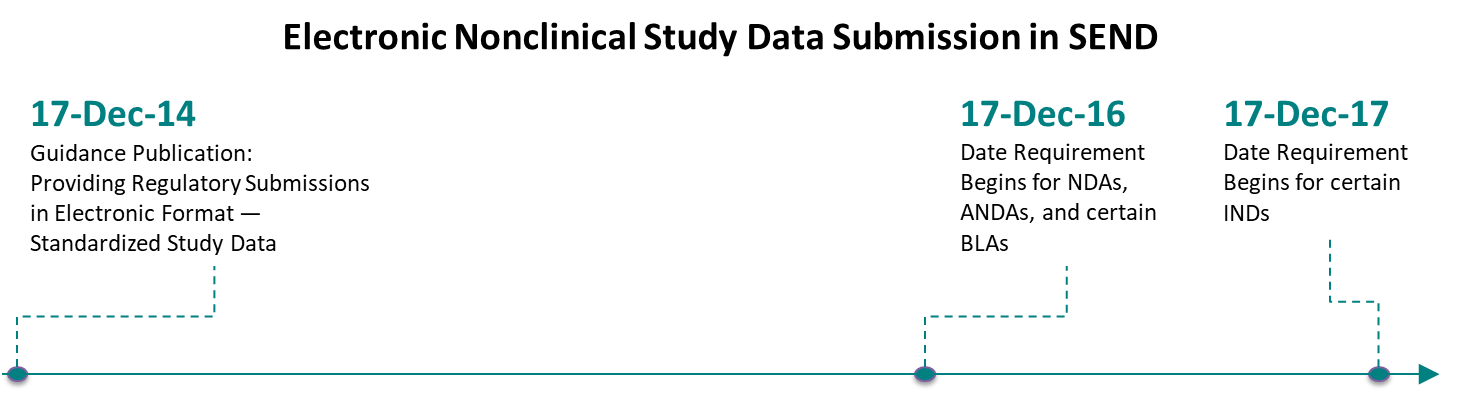
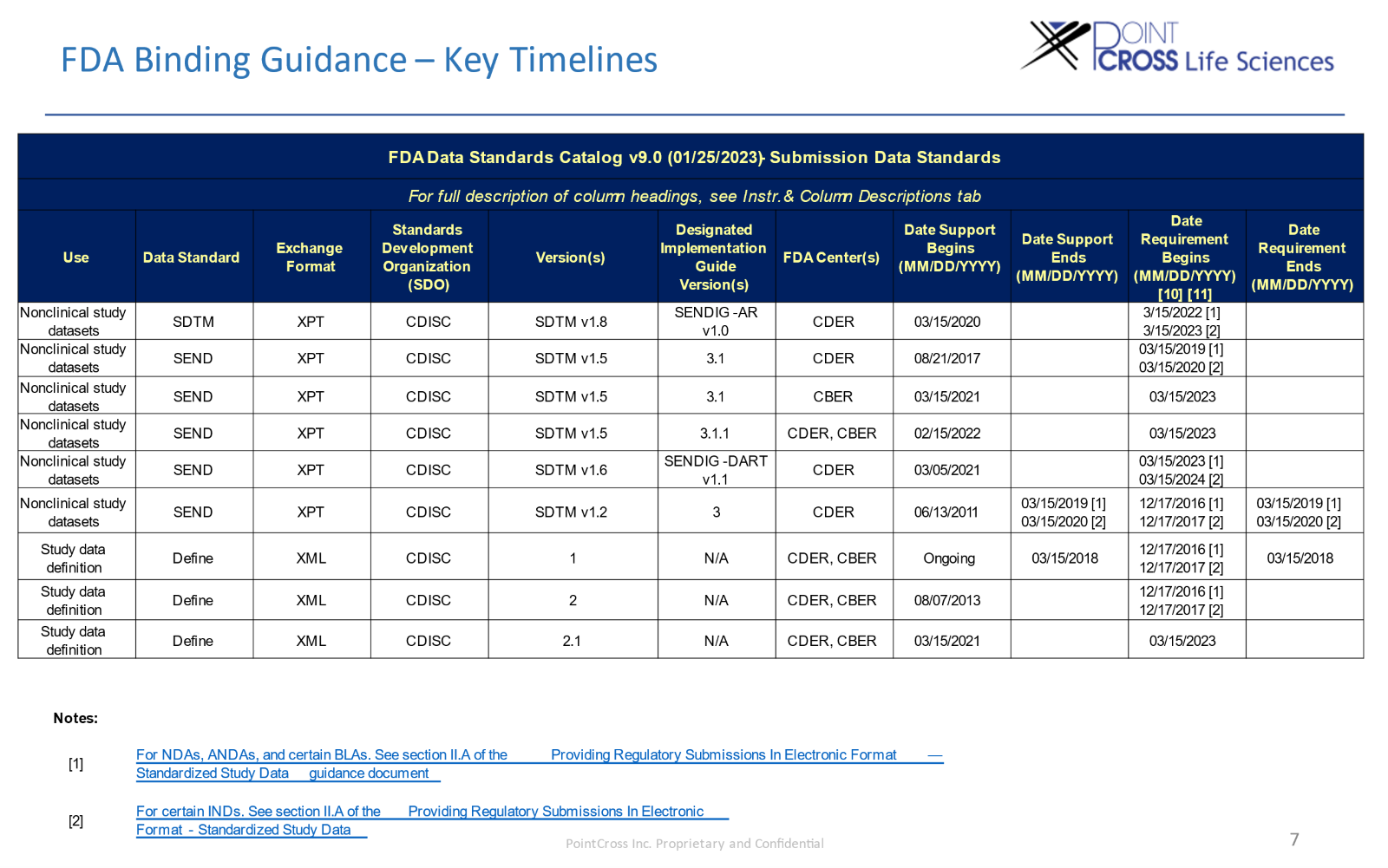
FDA Binding Guidance – Key Timelines
What are SEND Datasets?
SEND is the format for eData submission of subject level as-collected data for nonclinical studies as a tabulated data model. The SEND format includes a well-structured data format and defined terms to maintain consistency in data across all nonclinical studies. FDA has accepted CDISC SEND as a format for submitting eData for nonclinical studies. CDISC releases the SEND IG versions periodically (latest is SEND IG v3.1, draft of 3.2 is in process) The CDISC SENDIG (SEND implementation guide) Version 3, 3.1 and 3.1.1 describes the standard for representing the study data for general toxicology and carcinogenicity studies, which have been put in place to ensure the quality and completeness of the submitted data.
SEND Datasets consists of:
- Verified collected data for planned and unplanned endpoints
- Original values and standardized results
- Study design, including treatments administered.
The purpose of SEND Datasets is Machine readable representation of Nonclinical data to facilitate review of the collected data using standard tools and reduce the effort and time required to prepare the submitted studies for the medical reviewers
The submission package must meet the Technical Conformance guide issued by FDA – specifically these will include:
- Conformance rules published by CDISC
- Business Rules from FDA CDER (Published, public comments received, updates possible)
- FDA Technical Rejection Criteria as it is applied to SEND
Benefits of SEND
The FDA reviewers and the Sponsors can both benefit from SEND.
- Provides potential for data mining, historical control, and data warehousing.
- A standard format facilitates effective data sharing and communication between the Sponsor and the CRO.
- The uniform vocabulary (harmonized terms) and presentation format of the data facilitate quick and efficient evaluation by removing the need to comb through hundreds of pages of data and reference many summary tables.
- More effective nonclinical data sharing between and within companies
- SEND data sets must be submitted with an accompanying nSDRG and Define.xml file.
- Define File — Data definition file for metadata contained in the SEND data set.
- nSDRG — The Nonclinical Study Data Reviewer Guide (nSDRG) describes assumptions, and explanations about SEND data to help Reviewers
Which CTD modules does SEND affect?
Only individual data (or individual animal data) from a non-clinical investigation are covered by the SEND format specification. The SEND criteria only apply to Module 4 (non-clinical study reports) of the eCTD because individual data is typically provided as an appendix to the study report. The installation of SEND has no impact on CTD Modules 2.4 (non-clinical overview) and 2.6 (non-clinical summaries).
SEND Data Standardization Services
To support the process of submitting SEND datasets, there are various SEND dataset services available. These services help sponsors and CROs to create and prepare SEND datasets for submission to the FDA.
PointCross is a prominent player in the SEND Data Standardization industry, holding a 25% share of the market. PointCross specializes in packaging nonclinical study data for submission to the FDA SEND dataset requirements, particularly for IND submissions.
PointCross’s Nonclinical Study Data Standardization (DS) Services transforms as-collected data, from LIMS or GLP sources, on study subjects in nonclinical studies into SEND formatted data with the structure organized according to the Trial Design prescribed by SEND IG, the terminology mapped to the Control Terminology list selected, and the data organized into Domains as specified in the selected SEND IG Version. These SEND files along with nSDRG Reviewer’s Guide, and the Define.XML files are packaged for submission to the FDA.
The goal is to make the process of submitting SEND datasets as easy and efficient as possible.
Study Data Reviewer’s Guide
During SEND data standardization, certain observations or assumptions are made in order to fit non- standardized data to the SEND model. These assumptions and observations are captured within the nSDRG along with:
- Study Design Summary – Overview of the general protocol used in the study.
- Trial Design Domain Overview – Summary of the Trial Arms, Trial Elements, and Trial Sets that were used in the study organized in a formatted table.
- Standards Used – Versions of the standards used for the SEND Implementation Guide, SEND Controlled Terminology, DEFINE.XML, FDA Specific SEND Validation Rules, and Pinnacle21 Validator.
- Non-standard Terminology – Terminology used in the study that is not present in one of the SEND Controlled Terminology codelists.
- Dataset Summary – List of the domains present in the SEND Validated dataset.
- Validation Outcome Summary – Overview of the number of errors and warning reported by the Pinnacle21 Validator and the PointCross Validator.
- Validation Method Used – Overview of the standards and validators used to confirm adherence to the SEND standards.
- Errors – List of all the errors present in the study according to the PointCross Validator and Pinnacle21 Validator along with an explanation.
- Warnings – List of all the warnings present in the study according to the PointCross Validator and Pinnacle21 Validator along with an explanation.
- Sponsor-Defined Standardization Descriptions – A summary of the assumptions and observations made during the data standardization process.
- Differences between SEND Datasets and Study Report – A summary of the content differences between the SEND datasets and study report.
- Nonstandard Electronic Data Submitted – A list of any data that is not yet covered by the SEND standard that has been submitted along with the dataset.
The format of the nSDRG is based on the PhUSE Nonclinical nSDRG guidelines, which can be found at: Nonclinical Study Data Reviewer’s Guide (nSDRG) Package – WORKING GROUPS – PHUSE Advance Hub
SEND Quality Control
The Quality Control (QC) process for SEND datasets involves a series of checks and verifications to ensure that the data is accurate, complete, and consistent. The first step in this process is to check the metadata, which includes information about the study design, sample size, and data sources. This information is used to ensure that the data is relevant and appropriate for the intended use.
Another important aspect of the SEND dataset QC process is the verification of any calculations or statistical analyses performed on the data. These calculations must be checked to ensure that they are correct and that the results are consistent with the data. This step is critical in ensuring the validity of the results and conclusions that are drawn from the data.
The SEND dataset QC process also includes a review of the study documentation, such as protocols, reports, and data dictionaries. This documentation provides important information about the study design, methods, and results, and must be consistent with the data. Any inconsistencies or discrepancies between the documentation and the data must be addressed.
One of the tools used for SEND dataset QC is “SEND ASSURE” by PointCross, a software application designed specifically for this purpose. PointCross provides a comprehensive set of features for checking the quality of SEND datasets, including data validation, metadata analysis, and study documentation review. It also provides an easy-to-use interface for managing the QC process and tracking progress.
SEND Submission
Once the SEND dataset is ready, the next step is to submit it to the FDA. The submission must follow the guidelines set by the FDA, including submitting a data package that includes the SEND dataset, study reports, and other relevant documents. The submission process must be completed within the time frame specified by the FDA to avoid any delays in the drug development process
When accuracy of data and SEND/FDA compliancy are ensured, the study is signed off for delivery to the client along with supporting collateral
Deliverables of a SEND Data Standardization Package includes:
- Zip package containing the validated SEND xpt files, define file in .XML and PDF, and define2-0-0.xsl stylesheet file
- PointCross Validation Report for FDA Specific SEND 3.1.1 validation rules
- PointCross Validation Report for FDA Specific Business Rules
- CDISC Validation Report FDA Specific Business Rules
- CDISC Define Validation Report
- Pinnacle21 Validation Report
- Pinnacle21 define Validation Report
- Nonclinical Study Data Reviewer’s Guide (nSDRG)
How will the FDA use SEND files?
The FDA utilizes SEND files in their assessment of the safety and efficacy of new drugs, biologics, and medical devices. The SEND datasets, which are submitted in a standardized format, provide the FDA with in-depth information about nonclinical studies. By using the data contained in SEND files, the FDA can make well-informed decisions about the approval of new products, considering both their potential risks and benefits, and ensuring that they meet all necessary regulatory requirements.
By requiring SEND datasets, the FDA helps ensure that nonclinical study data is consistent and of high quality.
How is Nonclinical SEND Generated?
Nonclinical studies are conducted to evaluate the safety and efficacy of new drugs or therapies. These studies typically involve animals and are designed to provide data on toxicity, pharmacokinetics, and other important factors. SEND data can be generated from a wide range of nonclinical studies, including toxicity studies, pharmacokinetic studies, and efficacy studies.
Generating high-quality SEND data can be challenging, as it requires careful mapping of data to the appropriate SEND domains and ensuring data quality and consistency.
PointCross generates SEND datasets that are 100% consistent with (and traceable to) the Study Report, including for data that may currently require custom domains, such as ADA. PointCross accurately regenerates the Trial Design and creates a Digital Study Report (DSR) to extract all essential metadata and summary tabulations for use in precisely populating SEND domains with with LIMS subject data and study metadata.
Benefits of using PointCross SEND Generation:
- Assured quality and 100% consistency with the GLP Study Report
- Lowest cost in the industry
- Rapid delivery timeline with expedited processing available
- Digital reconciliation ensures that SEND can regenerate all published summary tables in the Study Report
- SEND datasets can be visualized and further analyzed on Xbiom™ including for cross-study analysis and comparison in an indexed and searchable repository
- Digitized data is available in Study Report terminology (DSR) and in SEND-IG CT
- SEND dataset, Define.xml and nSDRG meet TCG rules and 100% no rejection guarantee based on technical rejection criteria
Conclusion:
SEND datasets and submissions play a critical role in the nonclinical study process. SEND dataset services and submission tools make the process of submitting SEND datasets as efficient as possible, so the drug development process isn’t delayed.
SEND is not only a regulatory requirement; it is anticipated to completely change how the FDA and the industry handle non-clinical data. For sponsor companies, it is a useful tool for managing, analyzing, and sharing non-clinical data both inside and outside of their organization. Organizations that manage non-clinical data need to get ready to adapt their processes and systems and train resources to satisfy this new regulatory obligation in order to quickly take advantage of the potential. Overall, it ought to benefit science in general, simplify data processing and assessment, and ultimately shorten time-to-market.

