What is SDTM?
SDTM (Study Data Tabulation Model) is one of the requirements for data submission to the FDA (U.S) and PMDA (Japan). To facilitate data collection, management, analysis, and reporting procedures, SDTM offers a standard for organizing and presenting data. Implementing SDTM helps with data warehousing and aggregation, encourages mining and reuse, makes sharing easier, supports due diligence and other crucial data review tasks. It enhances the regulatory review and approval process. Additionally, SDTM is utilized in pharmacogenomics and genetics investigations, medical devices, and non-clinical data (SEND).
Role of SDTM
The SDTM is the accepted format for tabulation data submission to the FDA. The SDTM was designated in 2011 as the standard format for reporting data for non-clinical trials after being declared the standard format for data submission for clinical research in 2004. Data managers must become proficient in SDTM to ensure seamless data submissions, because clinical and nonclinical data submissions must follow the SDTM standard.
PointCross releases Xbiom™ SDTM Generator
PointCross provides dashboards for curating and integrating auxiliary studies and biomarker assays. In order to automatically produce SDTM datasets with the chosen Implementation Guide (IG) version and Controlled Terminology (CT) version from EDC and eCRF data stored in a F.A.I.R. Unified Data Model (UDM).
SAP prescribes analysis on periodic EDC data lockouts; however, translational requirements are frequent or ad hoc to fulfill the analysis of bench to bedside data. To keep an ever fresh and curated data picture of the trial in the UDM, it is simple to continuously curate new patient and visit data using Xbiom.
The UDM serves many essential functions:
- Search, find, access, and select stratified cohorts and their data
- Analyze cohorts or subjects and generate TFL’s on demand
- Generate SDTM with selected IG and CT on demand
The Xbiom SDTM generator has uses for clinical Ops, biostatisticians, and biometricians. A curated UDM and SDTM can be produced by a typical read cycle of the EDC and biomarkers in less than 24 hours. This can save time, become a vital tool for the biometrics team, and help with the quality testing and data validation when used with the eDataValidator, which is also a component of Xbiom.
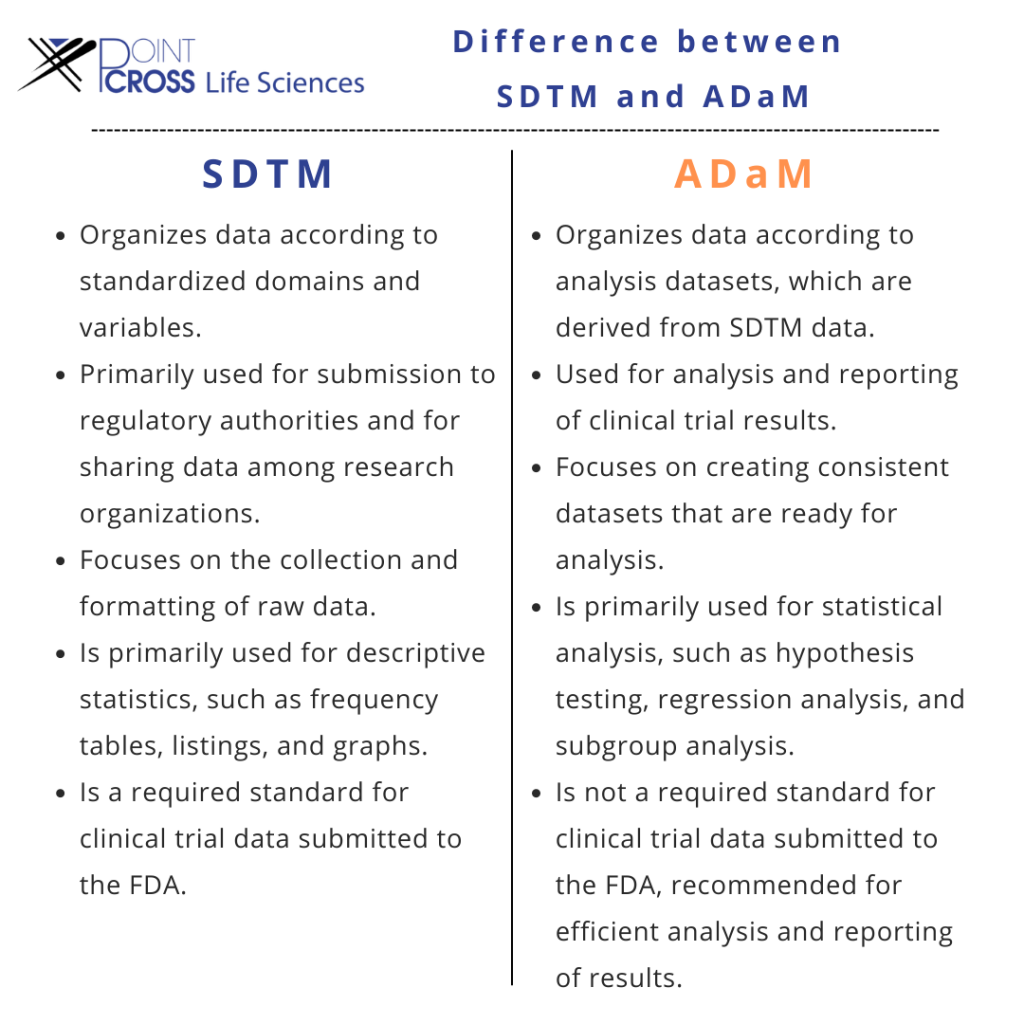
What is the difference between SDTM and ADaM?
Two important standards are supported by CDISC: SDTM (Study Data Tabulation Model) and ADaM. (Analysis Data Model). Although the adoption of CDISC standards may increase development costs, you can reduce those costs by choosing the appropriate technology and implementation strategy. The adoption of CDISC standards not only simplifies procedures but also stimulates innovation, efficiency, data quality, reduces costs, and boosts predictability.
In comparison to SDTM and SEND, CDISC’s Analysis Data Model (ADaM) is a little different. Although the model is less prescriptive, it still consists of a core model and an implementation guide to create datasets that are ready for analysis within specific limitations established by the model, more variables may be added. This provides a level of standardization that enables it to be easily understood by reviewers while giving it the flexibility to be used for any type of study.
SDTMIG – How is it different from SDTM?
SDTMIG is designed to provide guidance for the organization, structure, and format of standard clinical trial tabulation datasets, whereas SDTM provides a standard model for organizing and formatting data for human and animal trials. Although not limited to usage in regulated submissions, it was created to support data submitted to regulatory authorities like the US Food and Drug Administration (FDA). As stated in the FDA’s Data Standards Catalog for NDA (New Drug Applications), ANDA (Abbreviated New Drug Applications), and some BLA (Biologics License Applications) submissions, SDTM is one of the standards that sponsors are required to use.
With reference to a particular SDTM model, a SDTM Implementation Guide (SDTMIG) has been created. SDTM, on the other hand, builds on each new release of the model. The models can therefore be used with older hardware. For instance, SDTMIG-AP v1.0 was created with SDTM v1.4 in mind, but it can be used with SDTM v1.7 in a submission.
Here are some of the commonly asked questions regarding SDTM:
What are SDTM datasets?
SDTM datasets are tables of data that are created according to the SDTM standards and used to store information about the study participants, their visits, treatments, and events.
What are the safety domains in SDTM?
The safety domains in SDTM include the Adverse Event (AE), Concomitant Medication (CM). These domains capture information related to the safety of study participants, such as adverse events they experienced, medications they took, and their demographic information.
What are efficacy datasets in SDTM?
Efficacy domains in SDTM include Endpoints, Laboratory (LB), and Vital Sign (VS) domains. These domains capture information about the effectiveness of the study intervention, such as the endpoint results, laboratory test results, and vital signs of the participants.
What is the difference between CDASH and SDTM?
CDASH stands for Clinical Data Acquisition Standards for Clinical Research and is a standard for collecting and submitting clinical trial data. The difference between CDASH and SDTM is that CDASH focuses on the collection of data from clinical trials, while SDTM focuses on the organization and formatting of the data for submission.
How to do SDTM mapping?
SDTM mapping is the process of converting the raw clinical trial data into the standardized SDTM format. This process involves matching the data elements in the raw data with the standard SDTM data elements, as well as transforming and cleaning the data to ensure it is accurate and consistent.
Why is SDTM required?
SDTM is required for the submission of clinical trial data to regulatory agencies, as it ensures that the data is organized and formatted in a consistent and standardized manner. This makes it easier for regulatory agencies to review and assess the data.
Why is SDTM important?
SDTM is important because it helps to ensure the quality and accuracy of clinical trial data, as well as the consistency of the data across multiple studies. The use of SDTM also helps to improve the efficiency of data review and analysis, as well as the speed of the regulatory review process.
What are SDTM statistics?
SDTM statistics refer to the statistical analysis of clinical trial data that has been organized and formatted using the SDTM. This includes the calculation of descriptive statistics, such as mean, median, and standard deviation, as well as inferential statistics, such as hypothesis testing and regression analysis. The purpose of SDTM statistics is to provide insights into the safety and efficacy of the treatments being studied in the clinical trial, and to help inform decision making about the study. The SDTM statistics are a critical component of the regulatory submission.
How to create SDTM datasets?
To create SDTM datasets, one must first understand the SDTM standards and then map the raw clinical trial data to the SDTM format. The SDTM specifications provide detailed information about the standard data elements, the format, and the values for each SDTM domain.
How to validate and review SDTM datasets?
To review SDTM datasets, one must assess the accuracy and completeness of the data, as well as the consistency of the data with the SDTM standards. One must also ensure that the SDTM datasets are consistent with the CDISC submission requirements.
What is SDTM define.xml?
SDTM define.xml is a document that provides the detailed definition and structure of an SDTM dataset. It is a key component of the CDISC (Clinical Data Interchange Standards Consortium) standards and is used in the preparation and submission of clinical trial data to regulatory agencies. The define.xml document serves as a comprehensive metadata file that describes the variables, values, and domains included in an SDTM dataset.
It provides information on the structure of the data, such as the data types and lengths of variables, as well as the relationships between variables and domains. The SDTM define.xml file is used by regulatory agencies to validate the data submitted during a clinical trial and to ensure that it meets the standards and requirements set forth by CDISC.
What is SDTM format?
The SDTM format is a tabular format that uses columns and rows to organize the data elements. The define.xml is a metadata file that provides information about the structure and content of the SDTM datasets. The epoch in SDTM refers to a specific time interval in the study, such as a treatment cycle or a follow-up period.
What is epoch in SDTM?
An epoch in SDTM refers to a defined time interval within a clinical trial. In the context of SDTM, epochs are used to represent different periods of time during which a subject is exposed to a particular treatment or study intervention. For example, an epoch might represent the period of time between the start of a treatment and the end of a follow-up period. The definition of epochs in SDTM is important because it helps to ensure that the data collected during the trial is organized and formatted in a consistent and standardized manner. This allows for more accurate and efficient data analysis, as well as easier regulatory review.
What is the difference between SDTM and SEND?
SEND stands for Standard for Exchange of Nonclinical Data and is a standard published by CDISC for organizing and formatting nonclinical trial data. The difference between SDTM and SEND is that SDTM focuses on organizing and formatting clinical trial data, while SEND focuses on organizing and formatting nonclinical trial data.
What is controlled terminology in SDTM?
Controlled terminology in SDTM refers to the use of standard and agreed-upon terms and definitions for certain data elements in the SDTM datasets. The use of controlled terminology ensures consistency and accuracy of the data across multiple studies.
What is the latest version of SDTM IG?
The latest version of SDTM IG is version 3.4, which was released in November 2021. The SDTM IG provides detailed information and guidelines for implementing the SDTM standards in clinical trials.
What is SDTM Automation?
SDTM automation refers to the use of technology to streamline the process of transforming raw clinical trial data into the standardized SDTM format. This involves automating tasks such as data mapping, terminology, normalization, validation, and conversion, to reduce manual effort and improve data quality.
What is CDISC SDTM?
CDISC SDTM (Study Data Tabulation Model) is a standardized format used in the pharmaceutical and clinical research industry to organize and present clinical trial data. It ensures consistency and enables data exchange and analysis across studies, promoting efficiency and transparency in research.

