Clinical trial data management is a complex process that requires the collection and analysis of vast amounts of data. To ensure data consistency, accuracy, and integrity, the pharmaceutical industry has developed the Standard Data Tabulation Model (SDTM). In this article, we will explore the significance of automation in clinical trials, particularly automated data mapping, and how it leads to streamlined clinical trial processes.
SDTM: An Essential Element in Clinical Trial Data Management
SDTM is a standard data model that defines the structure and content of clinical trial data. SDTM is one of the requirements for data submission to the FDA (U.S) and PMDA (Japan). It is used to organize and present clinical trial data in a standard format that can be easily understood by regulatory authorities, clinical researchers, and data analysts. SDTM datasets are used to summarize patient demographic and baseline information, adverse events, findings, and other relevant clinical trial data.
What is SDTM Automation?
SDTM automation refers to the use of technology to streamline the process of transforming raw clinical trial data into the standardized SDTM format. This involves automating tasks such as automated data mapping, terminology, normalization, validation, and conversion, to reduce manual effort and improve data quality in automating clinical trials.
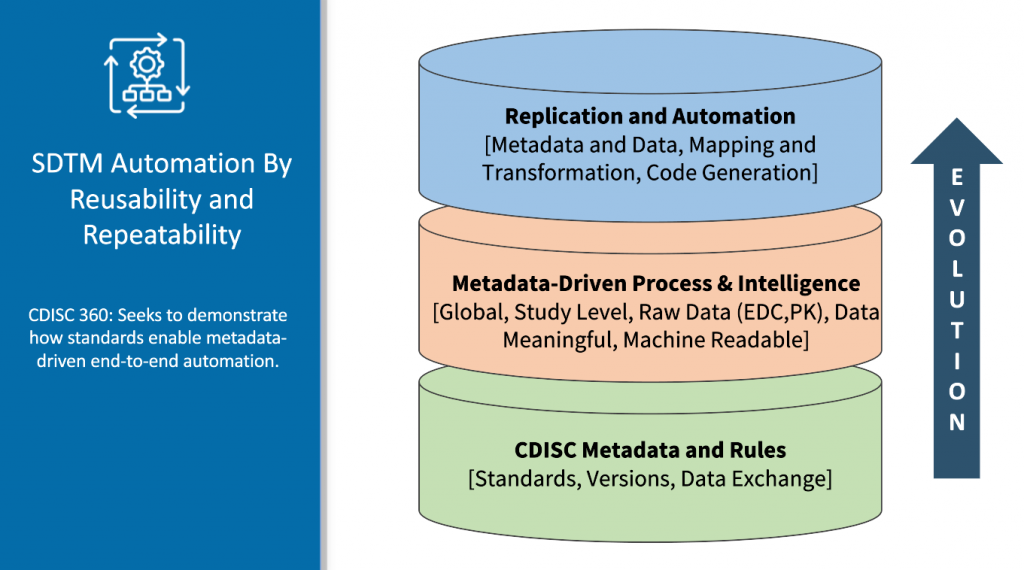
The Problem with manual data management
Clinical trials generate large amounts of data, which can be challenging to manage and analyze efficiently. On average it takes 6-8 weeks for SDTM mapping and generation. The problem arises in Quality and consistency issues, gaps in data, reconciliation, and all of these are still manually and programmatically done. However, even with SDTM in place, manual data management can be time-consuming and prone to errors. That’s where SDTM automation comes in.
To reduce the time required to generate SDTM datasets, automation has become a critical aspect of clinical trial data management. Automated SDTM generation uses software programs to map raw clinical trial data into standardized SDTM datasets. This process reduces the time required for data mapping and increases the accuracy of the generated datasets.
PointCross Automated SDTM Generation
PointCross is one of the leading providers of automated SDTM generation services. With its advanced algorithms and Xbiom’s Smart Transformer capability, PointCross can automate the process of mapping raw clinical trial data into SDTM datasets.
PointCross also could help identify inconsistencies in data and reduce time in generating SDTM. The Xbiom Suite accurately identifies and maps relevant data into the appropriate SDTM domains, minimizing manual efforts. As a result, biostatisticians and programmers can focus more on quality assurance (QA) tasks instead of coding-intensive processes, thus streamlining clinical trial data management.
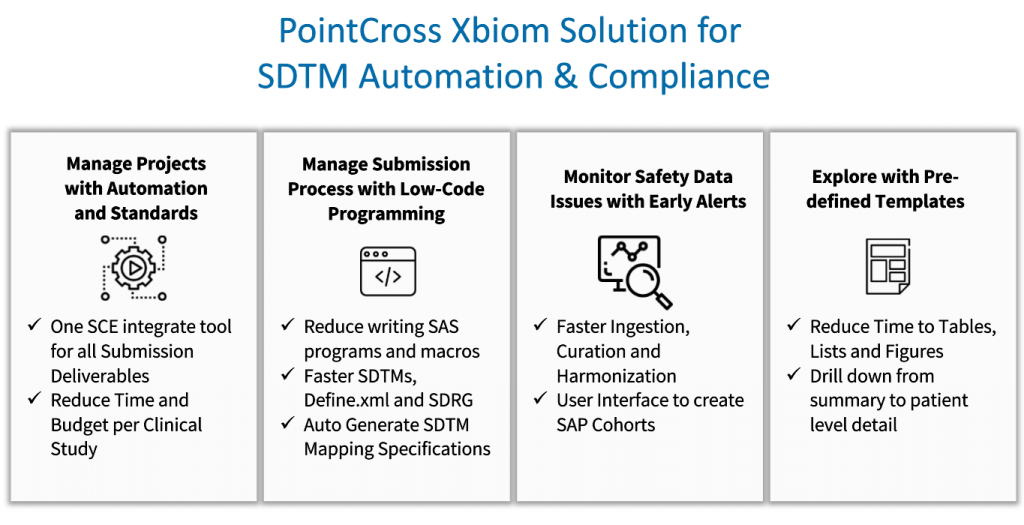
Learn more about EDC Raw Data to SDTM Curation, Mapping and Automation with the Xbiom Tool
Benefits of Automated SDTM Generation
SDTM automation helps streamline the process of transforming raw clinical trial data into the standardized SDTM format, reducing manual effort, and improving data quality. Automated SDTM generation offers several benefits to clinical trial data management, including:
- Automation in Clinical Trials: Increased Efficiency: Automated SDTM generation reduces the time required to map raw clinical trial data into SDTM datasets. This can save valuable time and increase efficiency in the clinical trial process.
- Automated Data Mapping: Improved Accuracy: Automated SDTM generation eliminates the potential for human error in the data mapping process. This increases the accuracy of the generated SDTM datasets, ensuring data consistency and integrity.
- Clinical Trial Automation: Compliance with Regulations: SDTM datasets are used to support regulatory submissions and must meet specific regulatory requirements. Automated SDTM generation ensures that generated datasets are in compliance with regulatory standards.
- SDTM Clinical Trials: Reduced Costs: Automation can result in cost savings by reducing the need for manual data management, increasing efficiency, and reducing the risk of errors.
How to Implement SDTM Automation
- Identify your Data Management Needs: Assess your data management requirements and determine which tasks can be automated.
- Choose the Right Tools: Choose SDTM automation tools that meet your specific needs and have the necessary features and capabilities.
- Implement Data Mapping: Map your raw data to the SDTM format using automated tools to simplify the process.
- Validate and Convert Data: Use automated tools to validate and convert data into the SDTM format, ensuring accuracy and completeness.
- Monitor and Maintain: Regularly monitor your automated processes and make necessary updates and modifications to maintain data quality and accuracy.
PointCross helps to easily Implement SDTM Automation and ensure 100% reconciliation of the SDTM/SEND dataset, enhanced regulatory compliance, and reduce costs.
View Webinar on Harmonizing Raw Data with CDISC Standards to Streamline SDTM
In conclusion, SDTM is an essential element in clinical trial data management. Automated SDTM generation offers several benefits, including increased efficiency, improved accuracy, and compliance with regulatory requirements. PointCross is a leading provider of automated SDTM generation services, offering innovative software that reduces the time required to generate SDTM datasets and increases the accuracy of the generated datasets. By using automated SDTM generation, clinical trial data management processes can be streamlined, saving time, and increasing efficiency in the clinical trial process. It is an essential consideration for organizations involved in automating clinical trials.
Download PDF version of this article: Introduction to SDTM Automation: Streamlining Clinical Trial Data Management

