PhUSE CSS 2020 Poster
PhUSE CSS 2020 September 21 – 23, 2020
“A Process for Automated Reconciliation of SEND Datasets with Study Reports for Confident Submission and Review.”
Abstract
SEND datasets and study reports are created by different processes, with study reports having predefined groupings, terminologies, and reporting conventions. In our opinion, regulatory reviewers trust reports because they fall under GLPs, are audited by QA, and signed by the Study Director. To reconcile SEND datasets against study reports, we propose a process in which study report tables are converted to a machine-readable columnar format that includes all summary tabulations and subject level listings. 100% automated reconciliation of tables in study reports with regenerated summaries from SEND is then possible. Automated “reconcilers” can use semantic enrichment for controlled terminologies and compare mapping between predefined cohorts in the study report to those in the SEND trial design. We propose that this model will demonstrate to reviewers that SEND datasets accurately represent data in study reports.
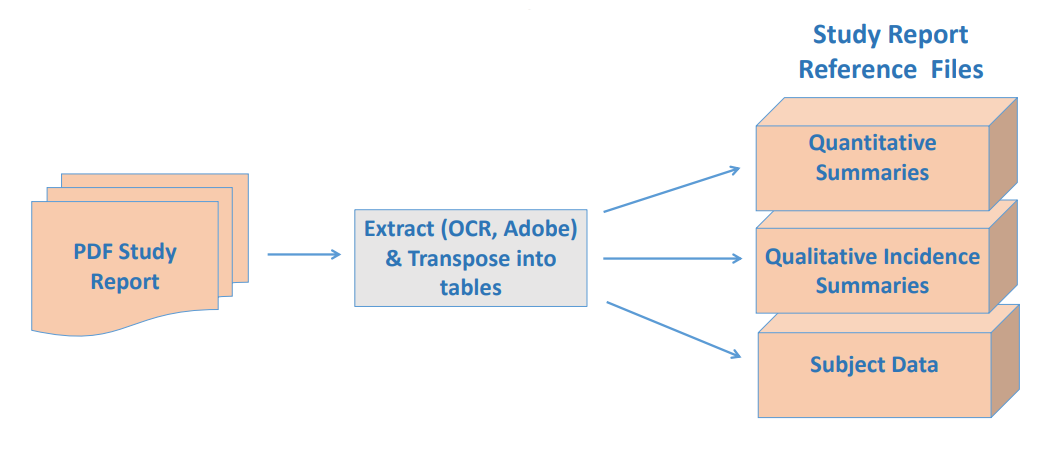
- Extract study report summary tables and subject-level data
into digital machine readable form
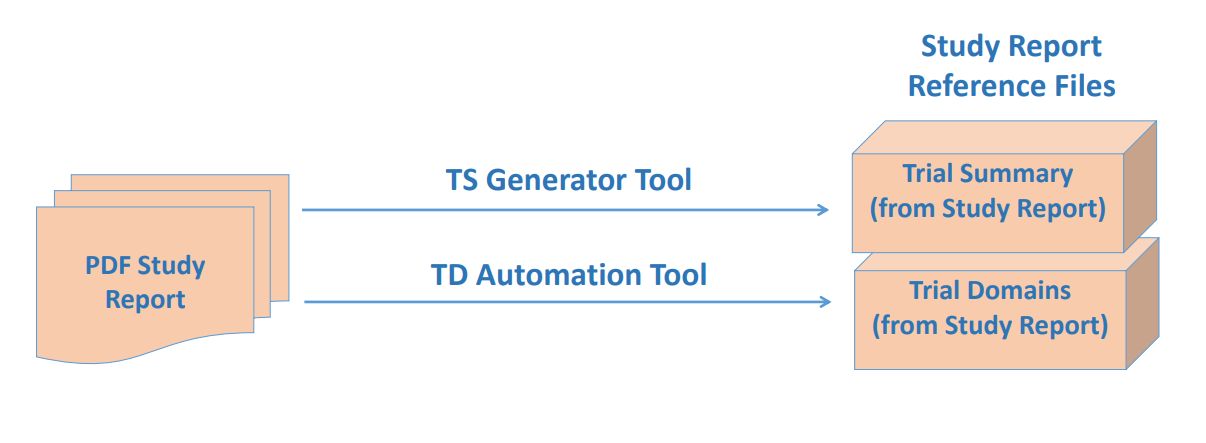
2) Generate Trial Summary and Trial Design ONLY from Study Report using automated tools
3) Regenerate group summary data from SEND dataset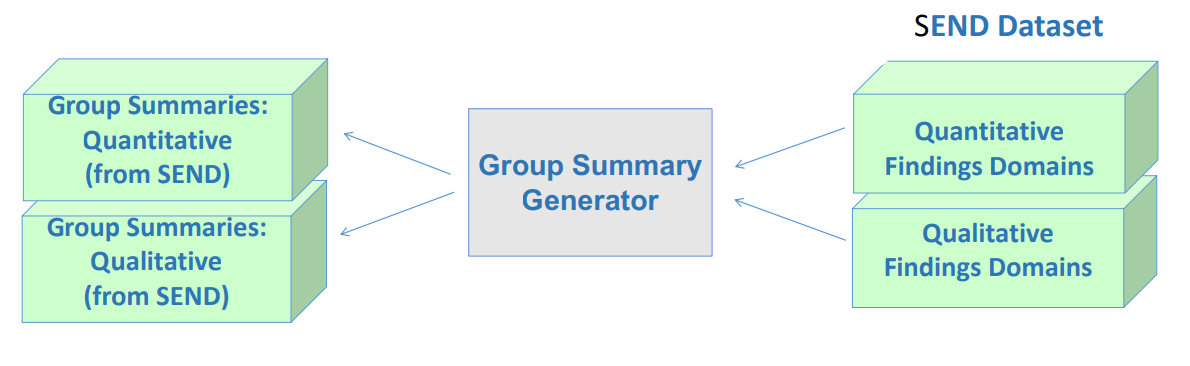
At this point in the process, there are several sets of machine readable files:
1. One set of summaries extracted from PDF study report
2. Another set of summaries regenerated from SEND dataset
3. TS and Trial domains generated from PDF study report
4. TS and Trial domains from SEND dataset
4) Use Comparator/ Reconciler tool to compare study report data with regenerated summary from SEND and SEND Trials and TS domains
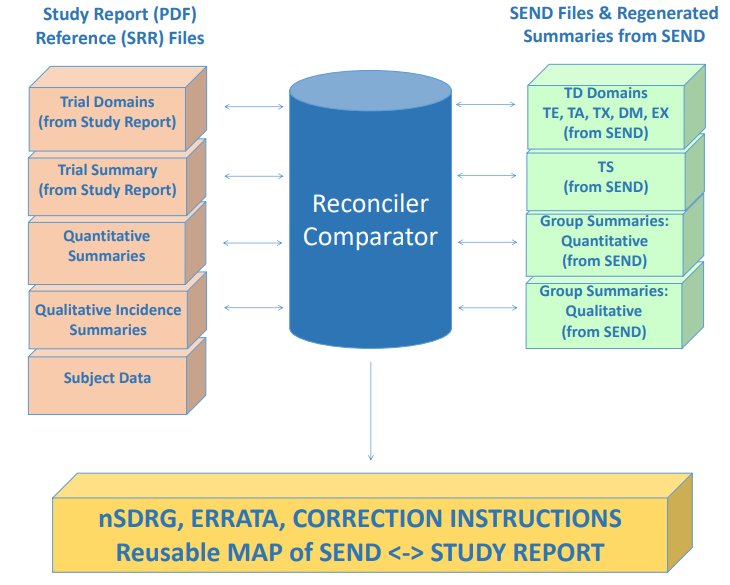
Notes:
• During steps 1 & 2, Study Report page # & section # are extracted for traceability of Study Report to it’s digital representation in Study Report Reference files.
• Additional information is extracted from Study Report text that is needed
for SEND but not part of Study Report summary tables.
• Subject level data can be extracted from Study Report appendices if necessary to resolve significant issues in SEND datasets.
• The output of this process includes human-readable files that identify inconsistencies between SEND dataset & Study Report, correction
instructions, notes for nSDRG & complete traceability between SEND dataset & Study Report.
CONCLUSIONS
According to FDA’s Technical Conformance Guide (Section 8.3), traceability allows the reviewer to understand and trace relationships between analysis results, single animal listings in the Study Report, and the tabulation data sets. Since SEND is generated independently of study reports, we believe that systematic and complete reconciliation with study reports is necessary. We have presented an automated method for 100% reconciliation that does not require any proprietary tools. This process can assure sponsors and reviewers that SEND datasets accurately represent data in the final study report
PointCross presented and was awarded Best Poster In the Industry category for our poster. Click the link below to view the poster.

