Clinical trials are an essential part of the drug development process, allowing researchers to evaluate the safety and effectiveness of new treatments. These trials involve testing a new medication or therapy on humans, usually in several phases, to determine its efficacy, safety, and potential side effects. In this blog post, we’ll explore the basics of clinical trials, including their purpose, types, and phases.
Introduction to Clinical Trials
A clinical trial is a research study conducted on humans to test the safety and effectiveness of a new medical treatment. This treatment can be a drug, vaccine, device, or any other intervention that has the potential to improve human health. Clinical trials aim to answer specific research questions and are designed to generate scientific evidence that can inform clinical practice.
Why are clinical trials important?
- Ensuring safety and effectiveness of new treatments
- Regulatory requirements for approval
- Advancing medical knowledge and improving patient care
- Patient benefits and opportunities for participation
Clinical trials are critical to the drug development process, as they provide researchers with valuable information about a new treatment’s safety, efficacy, and side effects. Without clinical trials, new treatments would not be approved for use, as regulators require evidence of their safety and effectiveness before they can be prescribed to patients.
Moreover, clinical trials help advance medical knowledge and improve patient care by identifying new treatments for diseases that currently have limited treatment options. Clinical trials also offer patients an opportunity to participate in cutting-edge research and potentially receive treatments that may benefit their health.
Types of clinical trials
Some of the most common types of clinical trials include:
- Interventional Trials: Interventional trials, also known as treatment trials, are designed to evaluate the safety and effectiveness of new treatments, interventions, or therapeutic approaches. In these trials, researchers actively intervene by administering a specific treatment or intervention to participants and then assess its outcomes. This could involve testing new drugs, medical devices, surgical procedures, behavioral therapies, or other interventions. Interventions are usually compared to a control group, which can receive a placebo, standard treatment, or an alternative treatment. The primary goal is to determine the efficacy and potential side effects of the intervention being studied.
- Observational Studies: Observational studies, as the name suggests, involve observing participants. Researchers collect data on participants’ health outcomes, risk factors, behaviors, or exposures and analyze the information to identify patterns, associations, or correlations. Observational studies are useful for investigating long-term effects, examining population health trends, identifying risk factors, or exploring the natural course of a disease.
Both interventional trials and observational studies play crucial roles in clinical research. Interventional trials provide evidence for the efficacy and safety of new treatments, helping guide clinical practice and the approval of therapies. Observational studies, on the other hand, provide valuable insights into real-world scenarios, contribute to generating hypotheses, and identify areas for further investigation. The combination of these two types of studies allows researchers to gather comprehensive and reliable evidence in healthcare and medical research.
What happens in a clinical trial or study?
In a clinical trial or study, researchers aim to evaluate the safety and effectiveness of a medical intervention, such as a new drug, treatment, or medical device. The process typically begins with preclinical research and laboratory studies to assess the intervention’s potential and establish a basic understanding of its effects. If the initial results are promising, the intervention progresses to human clinical trials.
These trials are conducted in multiple phases, starting with a small group of healthy volunteers or patients and gradually expanding to larger populations. Throughout the trial, participants are closely monitored, and data is collected to assess the intervention’s impact on their health outcomes. The study follows a carefully designed protocol, including specific criteria for participant selection, randomization, and control groups, to ensure rigorous and unbiased results. The trial concludes with data analysis, interpretation, and reporting of the findings, which contribute to scientific knowledge and inform regulatory decisions about the intervention’s approval, safety, and effectiveness.
Unlock the power of clinical interim study monitoring with PointCross’ innovative solution, Xbiom™ Insights. Our solution integrates real-time EDC patient data and biomarker data from bio-samples, providing valuable insights for biotech developing biologic therapeutics. Accelerate your research and development with Xbiom™ insights. Learn more on Monitoring Clinical Trials with Interim Data
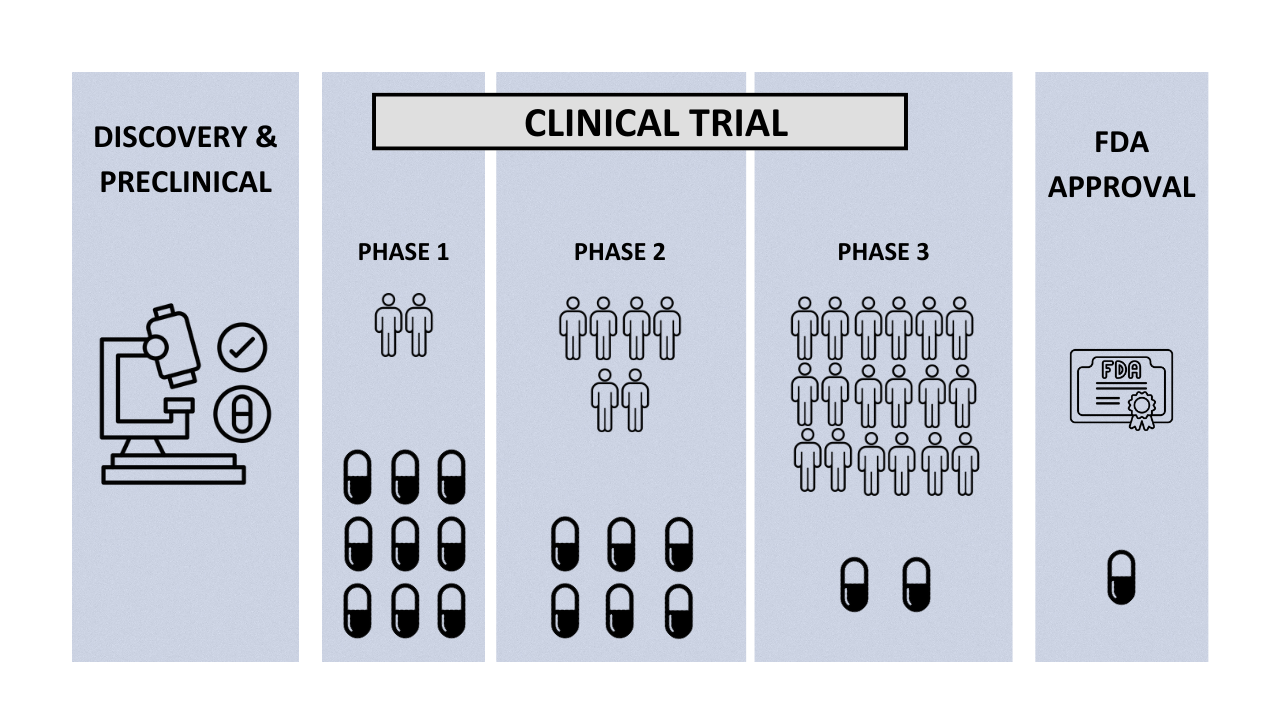
Phases of clinical trials
Clinical trials are conducted in several phases, each with a specific purpose and study design. These phases include:
- Phase 1: In this phase, a small group of healthy volunteers receives the new treatment to determine its safety, dosage, and potential side effects.
- Phase 2: In this phase, the treatment is given to a larger group of patients to evaluate its effectiveness and optimal dosage.
- Phase 3: In this phase, the treatment is given to a larger group of patients to confirm its effectiveness and monitor any side effects.
- Phase 4: This phase takes place after the treatment has been approved for use and aims to monitor its long-term safety and effectiveness.
Why are clinical trials done?
Clinical trials are conducted to evaluate the safety and effectiveness of new drugs, treatments, or medical interventions before they can be widely used. These trials provide vital data that regulatory authorities rely on to assess and approve interventions for public use. By testing innovative approaches, clinical trials drive medical progress, improve patient care, and expand our understanding of diseases. They prioritize patient safety by closely monitoring participants for adverse effects. Clinical trials also contribute to evidence-based medicine, guiding healthcare professionals in making informed treatment decisions. Furthermore, they offer patients access to potentially beneficial therapies and pave the way for continuous improvement in medical practices.
If you’re involved in clinical trials, translational, and biomarker research, PointCross offers comprehensive solutions to enhance your processes. Our expertise includes monitoring ongoing clinical trial EDC data and biomarker assays, facilitating real-time analysis and visualization. With our platform, you can efficiently search, select, and compare highly stratified cohorts based on clinical endpoints, biomarker data, and more. Enhance your research capabilities today. Visit INSIGHTS – Clinical Biomarker to learn more.
Commonly Asked FAQs in Clinical Trials: What You Need to Know
What is a clinical trial?
A clinical trial is a research study conducted on humans to evaluate the safety and effectiveness of new medical treatments, interventions, or procedures.
Why are clinical trials important?
Clinical trials are crucial for advancing medical knowledge, developing new treatments, improving patient care, and finding potential cures for diseases.
Who can participate in a clinical trial?
Eligibility criteria vary for each trial, but typically, participants are selected based on factors such as age, medical history, and specific health conditions.
How can I participate in clinical trials?
If you want to dive deeper into the world of clinical trials, visit ClinicalTrials.gov. This official database provides comprehensive information about ongoing clinical trials, including their purpose, design, eligibility criteria, and locations. Explore the site to learn about the latest research studies and discover opportunities to participate in clinical trials that can shape the future of medicine.
What are the different phases of a clinical trial?
Clinical trials have four phases: Phase 1 tests safety and dosage, Phase 2 evaluates effectiveness, Phase 3 assesses safety and efficacy in larger groups, and Phase 4 monitors long-term effects after approval.
How long do clinical trials typically last?
The duration of clinical trials can vary widely, ranging from a few months to several years, depending on the study’s objectives and design.
What are the potential risks and benefits of participating in a clinical trial?
Potential risks include side effects, discomfort, or the treatment being ineffective. Benefits may include access to new treatments, close medical monitoring, and contributing to scientific knowledge
How are participants protected in a clinical trial?
Participants are protected through informed consent, strict ethical guidelines, review boards, and regulatory oversight to ensure their rights, safety, and privacy.
Will I receive any compensation for participating in a clinical trial?
Some clinical trials provide compensation to participants for their time, travel expenses, or inconvenience, but this varies depending on the trial’s sponsor and design.
What is informed consent, and why is it necessary?
Informed consent is a process where participants are provided with comprehensive information about the trial, its risks, benefits, and their rights, allowing them to make an informed decision before participation.
What happens if I experience side effects during the trial?
If you experience side effects, the trial’s medical staff will provide appropriate care, monitor your condition, and may adjust the treatment or, if necessary, discontinue your participation.
Can I leave a clinical trial before it is completed?
Yes, you have the right to withdraw from a clinical trial at any time, for any reason, without any negative consequences or impact on your future medical care.
Will I receive experimental treatment or a placebo?
It depends on the specific trial design. Some participants receive experimental treatment, while others may receive a placebo or standard treatment for comparison purposes. This information is disclosed during the informed consent process.
How is the effectiveness of the treatment assessed in a clinical trial?
The effectiveness of a treatment is assessed through careful monitoring of participants’ health outcomes, comparing the treatment group to control groups, and analyzing the data collected during the trial.
How are clinical trial results communicated to participants?
Clinical trial results are typically shared with participants through various means, such as written reports, newsletters, or study websites. Some trials also hold participant meetings to discuss the findings.
Are there any costs involved in participating in a clinical trial?
In many cases, the costs associated with participating in a clinical trial, such as study-related medical tests and treatments, are covered by the trial sponsor. However, it is essential to clarify this with the trial organizers beforehand.
How can I find clinical trials that I might be eligible for?
You can search for clinical trials through online databases, research institutions, hospitals, or by consulting with healthcare providers specializing in your medical condition.
What happens after the clinical trial is completed?
After a clinical trial is completed, the results are analyzed, and if the treatment proves safe and effective, it may seek approval from regulatory authorities for widespread use.
How are clinical trials regulated and monitored?
Clinical trials are strictly regulated and monitored by ethical review boards, government agencies, and independent committees to ensure participant safety, scientific integrity, and adherence to regulations.
Can I participate in more than one clinical trial at a time?
Participating in multiple clinical trials simultaneously is generally not allowed due to potential complications and conflicting treatment protocols. It is important to discuss this with the trial organizers.
Are there any specific eligibility criteria for participation in a clinical trial?
Each clinical trial has specific eligibility criteria based on factors like age, gender, health status, and medical history. These criteria are set to ensure the trial’s safety and effectiveness and may vary for each study.
Conclusion
Clinical trials are a crucial step in the drug development process, providing researchers with important information about the safety and efficacy of new treatments. These trials offer patients an opportunity to participate in cutting-edge research and potentially benefit from new treatments. Understanding the basics of clinical trials is essential for patients, healthcare professionals, and anyone interested in advancing medical knowledge and improving patient care.
Download PDF version of this article: Clinical Trials: Understanding the Basics, Types, and FAQs

