Table of Contents
In the fast-paced world of clinical research, managing metadata effectively is crucial. That’s where the Xbiom Metadata Management Repository (MDR) by PointCross comes in, offering a comprehensive solution for both clinical and nonclinical metadata needs. In this blog, we’ll delve into why metadata management is so important in clinical research, explore the standout features of Xbiom MDR, and show how this tool can improve data integration, regulatory compliance, and overall research efficiency.
The Importance of Metadata Management Repository in Clinical Research
Metadata management plays a crucial role in clinical research, ensuring that data is accurately described, easily accessible, and consistently maintained across various stages of research and development. Effective metadata management repository helps streamline data integration, facilitates regulatory compliance, and enhances the overall quality of data analysis and reporting.
Xbiom: A Comprehensive Solution
Xbiom by PointCross stands out as a powerful tool designed to meet the rigorous demands of clinical and nonclinical research environments. This solution offers a range of features tailored to optimize metadata management, including:
Centralized Metadata Repository
1. Centralized Data Storage Xbiom-MDR provides a centralized repository for all metadata, ensuring that data definitions, standards, and mappings are stored in one accessible location. This centralization facilitates consistency and integrity across data sets, reducing the risk of discrepancies and errors.
Scalability and Flexibility
2. Scalable Architecture The system is built to handle large volumes of data, accommodating the expansive needs of clinical and nonclinical research projects. Its scalable architecture ensures that as data grows, the system can expand seamlessly to meet increasing demands.
3. Flexible Configuration Xbiom-MDR’s flexible design allows for easy adaptation to evolving regulatory requirements and industry standards. This ensures that the system remains relevant and effective, regardless of changes in the regulatory landscape.
Enhanced Data Integration
4. Seamless Data Integration Xbiom-MDR excels at integrating data from various sources, including clinical trials, laboratory data, and electronic health records (EHRs). This capability is crucial for comprehensive data analysis and supports informed decision-making by providing a holistic view of all relevant data.
5. Interoperability The system supports interoperability with other data management systems and tools, ensuring that data can be easily shared and utilized across different platforms. This interoperability enhances collaboration and data sharing among research teams and institutions.
Regulatory Compliance
6. Built-in Compliance Features Xbiom-MDR is designed with regulatory compliance in mind, adhering to standards such as those set by the Clinical Data Interchange Standards Consortium (CDISC). These built-in compliance features simplify the process of meeting regulatory requirements, reducing the burden on research teams.
7. Audit Trails The system includes comprehensive audit trails, providing detailed records of all changes and updates made to the metadata. This feature is essential for maintaining transparency and accountability and meeting regulatory audit requirements.
User-Friendly Interface
8. Intuitive User Interface Despite its advanced capabilities, Xbiom-MDR offers an intuitive and user-friendly interface. This ease of use ensures that researchers and data managers can navigate the system efficiently, reducing the need for extensive training and allowing users to focus on their core tasks.
9. Customizable Dashboards The platform features customizable dashboards that allow users to tailor their workspace according to their specific needs and preferences. This personalization enhances productivity and makes it easier to access the most relevant information quickly.
Advanced Analytics and Reporting
10. Powerful Analytics Tools Xbiom-MDR includes advanced analytics tools that enable detailed data analysis and visualization. These tools help researchers identify trends, patterns, and insights that can drive better decision-making and more effective research outcomes.
11. Comprehensive Reporting The system provides comprehensive reporting capabilities, allowing users to generate detailed reports that meet both internal and regulatory requirements. These reports can be customized to include specific data points and insights, making them valuable tools for communication and compliance.
Security and Access Control
The Security and Access Control component ensures that all data within Xbiom is protected, and that access is controlled based on user roles and permissions. This component includes authentication mechanisms, encryption, and audit trails to maintain data integrity and security.
Security and compliance are integral to Xbiom’s architecture. The Security and Access Control (SAC) component ensures that data is protected through encryption, role-based access control, and detailed audit trails. The Compliance and Standardization Module ensures that all data management processes comply with relevant regulatory standards, thereby simplifying the compliance process for research organizations.
Xbiom Functional Components: Enhancing Metadata Management Repository
Xbiom by PointCross is a comprehensive solution designed to optimize metadata management in clinical and nonclinical research. Its functional components are divided into three primary categories:
- Metadata Repository
- Centralized Data Repository, and
- Regulatory Workflow.
Each of these components plays a crucial role in ensuring the system’s efficiency, compliance, and ease of use.
1. Metadata Repository
The Metadata Repository is the cornerstone of Xbiom, designed to store and manage all metadata related to clinical and nonclinical research. It includes:
- Metadata Definitions This sub-component captures detailed definitions of all data elements used in research, including variables, data types, permissible values, and relationships between data elements. These definitions ensure consistency and clarity across all datasets.
- Standards Management Xbiom supports various industry standards such as CDISC (Clinical Data Interchange Standards Consortium). This feature ensures that all metadata adheres to these standards, facilitating regulatory compliance and interoperability.
- Version Control The repository includes robust version control capabilities, allowing users to track changes to metadata over time. This ensures that historical data is preserved and that updates can be audited.
- Metadata Governance This aspect of the Metadata Repository ensures that all metadata is governed according to predefined policies and procedures. It includes roles and responsibilities, approval workflows, and audit trails to maintain data integrity and accountability.
2. Centralized Data Repository
The Centralized Data Repository complements the Metadata Repository by storing all actual data collected during research. Key features include:
- Data Integration The repository integrates data from various sources such as clinical trials, laboratory systems, and electronic health records (EHRs). This integration is facilitated by APIs, ETL processes, and other data integration technologies.
- Data Standardization Xbiom standardizes data upon entry, ensuring that all data conforms to predefined formats and standards. This standardization is crucial for accurate data analysis and reporting.
- Scalability The Centralized Data Repository is designed to handle large volumes of data, making it suitable for extensive research projects. Its scalable architecture allows for seamless expansion as data needs grow.
- Secure Storage Data security is a top priority, with the repository employing advanced encryption methods and access control mechanisms to protect sensitive information. Regular backups and disaster recovery plans ensure data availability and resilience.
3. Regulatory Workflow
The Regulatory Workflow component ensures that all research processes comply with relevant regulations and standards. It includes:
- Compliance Management This sub-component ensures adherence to regulatory standards such as GxP, FDA, and ICH guidelines. It includes tools for validating data and generating compliance reports.
- Workflow Automation Xbiom automates various regulatory workflows, from data collection and validation to submission and approval. This automation reduces manual effort and minimizes the risk of errors.
- Audit Trails Comprehensive audit trails are maintained for all actions within the system. These trails provide a detailed record of data access, modifications, and approvals, ensuring transparency and accountability.
- Reporting and Analytics The Regulatory Workflow component includes powerful reporting and analytics tools that help generate detailed reports for regulatory submissions. These reports can be customized to meet specific regulatory requirements and standards.
Integration and Collaboration
Xbiom’s functional components are designed to work seamlessly together, providing a cohesive and integrated environment for metadata management. The integration between the Metadata Repository, Centralized Data Repository, and Regulatory Workflow ensures that data flows smoothly through all stages of research, from collection to analysis and reporting.
Collaboration Tools Xbiom also includes collaboration tools that allow researchers, data managers, and regulatory personnel to work together effectively. These tools include shared workspaces, real-time data sharing, and communication platforms.
Xbiom: Metadata & Data Repository components
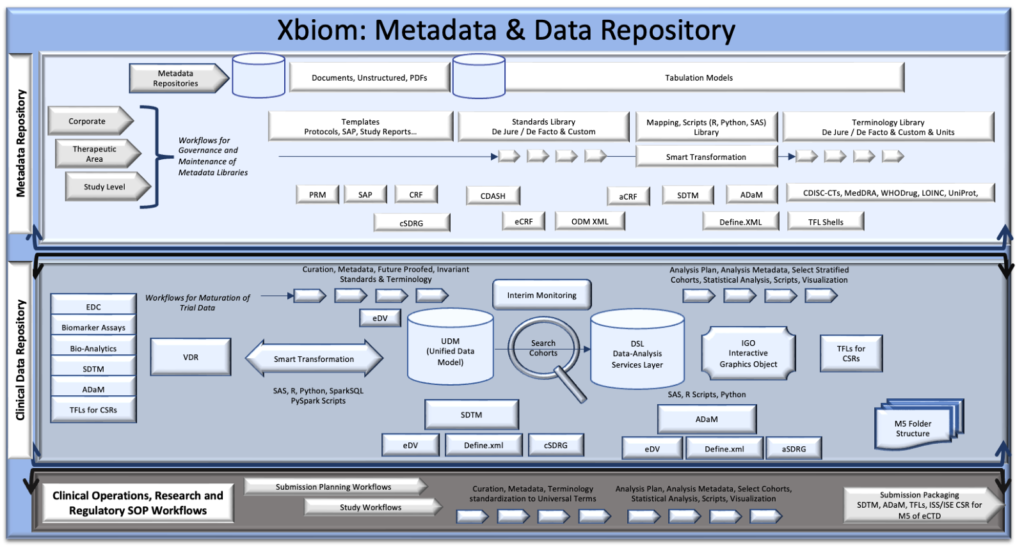
Impact on Clinical and Nonclinical Research
By leveraging Xbiom, organizations can significantly enhance their data management practices, leading to more efficient research processes and higher-quality outcomes. Key benefits include:
- Improved Data Quality: Centralized and standardized metadata management reduces the risk of data inconsistencies and errors, ensuring high-quality data for analysis.
- Faster Time to Insights: Efficient data integration and management streamline the research process, enabling quicker access to insights and accelerating the development of new treatments.
- Regulatory Confidence: Built-in compliance features provide assurance that all regulatory requirements are met, reducing the burden on research teams and minimizing the risk of non-compliance penalties.
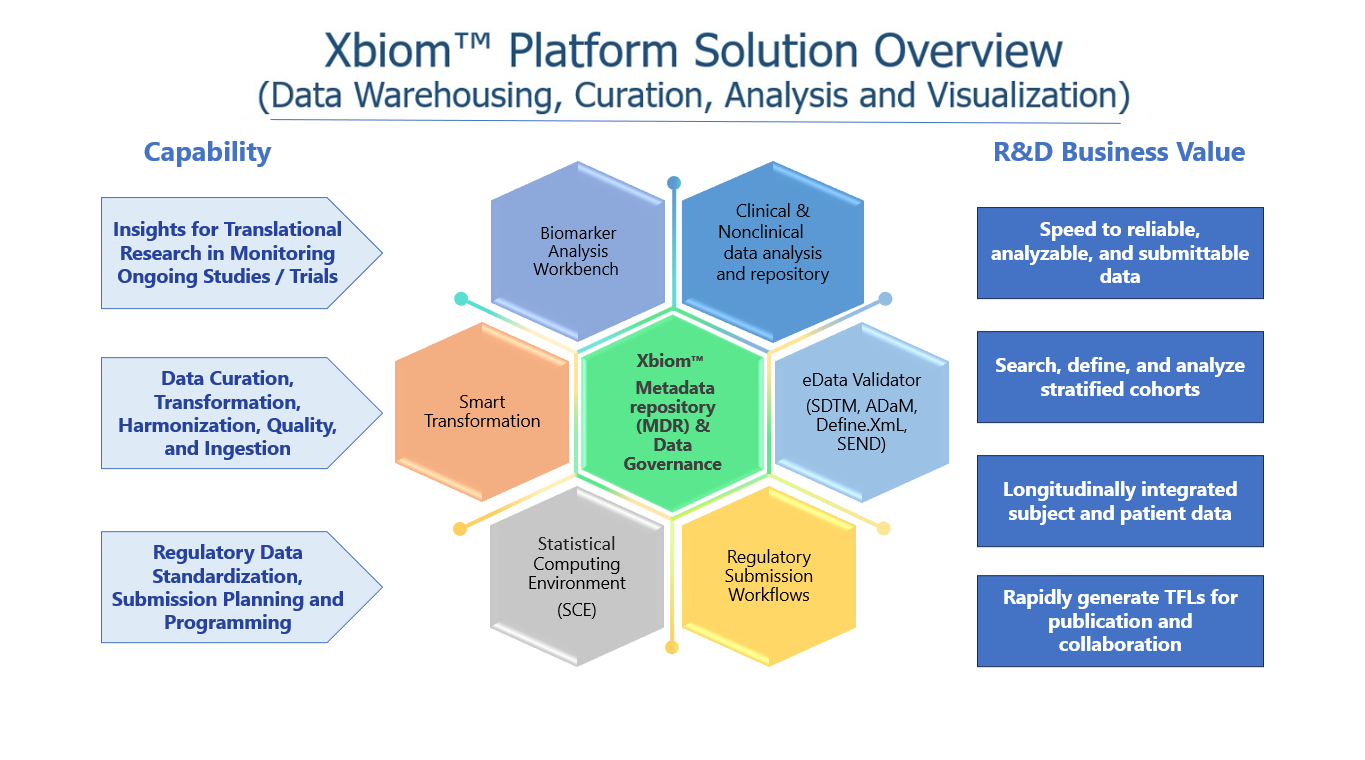
Conclusion
Xbiom MDR by PointCross represents a significant advancement in the realm of metadata management repository for clinical and nonclinical research. By integrating a robust Metadata Repository, a comprehensive Centralized Data Repository, and an efficient Regulatory Workflow component, Xbiom MDR addresses the critical needs of data accuracy, consistency, and compliance in research environments.
The Metadata Repository ensures that all data definitions, standards, and mappings are centralized and governed effectively, fostering consistency and integrity across datasets. The Centralized Data Repository facilitates seamless data integration, storage, and standardization, enabling researchers to handle large volumes of data efficiently and securely. Meanwhile, the Regulatory Workflow component automates compliance processes, maintains thorough audit trails, and generates regulatory reports, significantly reducing the administrative burden on research teams.
Through its scalable and flexible architecture, Xbiom MDR adapts to evolving regulatory requirements and industry standards, ensuring that research organizations remain compliant and efficient. The user-friendly interface and advanced analytics tools further empower users to navigate the system with ease, gain valuable insights, and make informed decisions based on high-quality data.
In summary, Xbiom MDR is a comprehensive solution that enhances the quality, compliance, and efficiency of metadata management in clinical and nonclinical research. By leveraging the capabilities of Xbiom MDR, research organizations can streamline their processes, ensure data integrity, and accelerate the development of new treatments, ultimately contributing to advancements in medical science and improved patient outcomes.

