In the troubled waters of modern drug development, where clinical insights are scattered across countless studies and siloed systems, researchers are searching for something that can safely carry them from fragmented data to unified intelligence, simply. They need a bridge.
AI promises to deliver the solutions to the problems that a historically averse to rapid change industry faces. But how does that mix with the reality that BioPharma operates in? How does an organization prioritize speed and efficiency while keeping data integrity and qualified responses from disparate data sources at the forefront?
The Reality: AI Is Coming Whether You’re Ready or Not
AI isn’t a trend. It’s the future of drug development. Period.
The question isn’t whether your organization will adopt AI, it’s whether you’ll adopt the ease and convenience of using a AI based Chat interface to your precious and complex clinical data. When you can Chat with your clinical trial data and let it draw you into its inner folds of tabulations and visualizations when you have the context but need the receipts, or proof.
Because here’s the fundamental issue: Generic AI platforms were never built with BioPharma in mind.

These systems were designed for consumer applications, trained on openly available internet content, and optimized for creative tasks in various data domains. They can never be exposed to BioPharma’s proprietary, secured data in the form of Clinical Trials or Nonclinical Studies. Posting these into an open AI LLM system is impossible without violating HIPAA, corporate secrecy, and leaking very valuable IP.
Meanwhile, traditional clinical data systems were built for use in this cloistered private enterprise environment. They store data effectively, make it available in standardized, or well-organized formats for analysis and insights. But it requires skill, training and experience to become proficient in the use of these scientific tools. They generate all the reports efficiently, but the tools can’t answer nuanced questions, or even imprecisely posed questions. They maintain compliance perfectly but can’t bridge users to the insights across studies.
The result? A fundamental architecture mismatch between what AI needs and what clinical systems provide.
Xbiom’s Universal Data Model (UDM) was built from day one to solve this exact problem. It is a unified repository designed specifically for LLM integration, clinical understanding, and cross-study intelligence.
The Silo Problem That AI Can’t Solve Alone
Right now, your critical insights are trapped:
- Regulatory documents live in one system
- Raw lab data sits in another
- Statistical analysis happens in an SCE
- Safety signals get buried in separate databases
- Cross-study patterns remain invisible because no single system can see them all
This isn’t a data volume problem. This is a data fragmentation problem.
You can have terabytes of perfect clinical data, but if users can’t see across study boundaries, understand clinical context, and retrieve with precision, then you’re still flying blind when making critical development decisions.
What BioPharma Actually Needs: RAG That Gets It
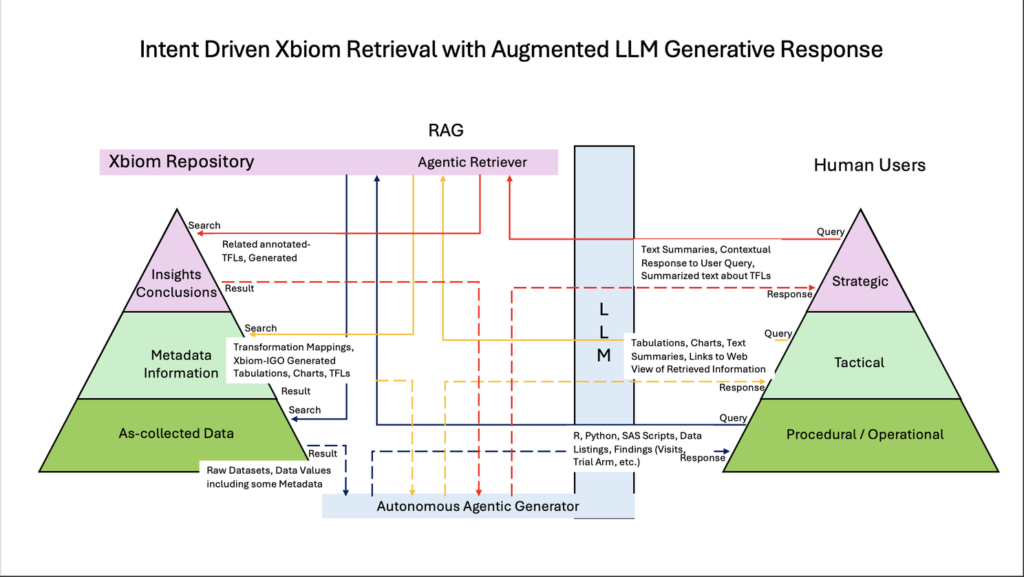
The breakthrough bridging a rigorous robust clinical data hub like Xbiom to an LLM AI system so that the user can converse with it through a Chat interface, but the responses are actually retrieved from the Xbiom Database’s extensive query mechanisms. Xbiom is Retrieval Augmented Generation (RAG) enabled, specifically built for such clinical research and development.
Here’s how it actually works:
Universal Data Model That Speaks Clinical
Xbiom’s unified repository doesn’t just store data, it responds to structured queries and responds to it. SDTM standards, clinical terminology, study structures, and regulatory requirements. The AI doesn’t guess about your data because it can actually use Xbiom to read your data through a contextually aware RAG system.
Intent Recognition That Gets Nuanced Queries
When a CMO asks, “What liver toxicity patterns emerge across our oncology portfolio?” the system:
- Identifies “liver toxicity” across all study terminology variants
- Recognizes “oncology portfolio” means specific therapeutic areas
- Searches across multiple study types and phases
- Returns results with the receipts, or proof of data, from Xbiom’s clinical, or nonclinical database
Cross-Study Intelligence That Breaks Down Silos
The magic happens when AI can synchronize with Xbiom to see patterns across:
- Preclinical safety studies → Clinical trial adverse events
- Biomarker data → Patient response patterns
- Protocol amendments → Operational learnings
- Historical studies → Current development decisions
Receipts, Not Hallucinations
Every answer traces back to actual study data. Every insight shows its work. Every recommendation includes the underlying evidence. No fabrication. No creative interpretation. Just verifiable clinical intelligence.
The Conversation Interface That Changes Everything
Forget complex menus and rigid query languages. The future is conversational:
Researcher: “Show me dose-response relationships for compound X across all studies”
Xbiom RAG: Retrieves data from 12 relevant studies, generates comparative visualization, highlights statistical significance patterns
CMO: “What safety signals should concern us for our diabetes pipeline?”
Xbiom RAG: Analyzes adverse events across portfolio, identifies emerging patterns, provides risk assessment with supporting data
Regulatory Affairs: “Pull submission-ready safety data for compound Y” Xbiom RAG: Compiles regulatory-grade datasets, ensures SDTM compliance, generates required tabulations with full audit trail
Natural language in. Actionable intelligence out. With receipts.
Business-Critical Impacts: Your Guide from Preclinical to Profitability
BioPharma sponsors face an epic journey: crossing from preclinical promise to profitable reality as fast as possible without compromising reliability. Here’s how RAG-enabled Xbiom becomes your trusted guide across these troubled waters:
Accelerated Decision Velocity: From Months to Minutes
The Hero’s Challenge: Critical development decisions currently take weeks or months because insights are trapped in departmental silos.
The Guide’s Solution: RAG enables instant cross-study intelligence that collapses decision timelines.
Business Impact:
- Protocol optimization based on learnings from your entire portfolio, not just current studies
- Go/no-go decisions supported by comprehensive evidence from day one, not after expensive delays
- Safety signal detection across therapeutic areas before they become program-killers
- Regulatory strategy informed by patterns from every submission in your history
Bottom Line: Speed isn’t recklessness, it’s having the right insights at the right moment to make confident decisions faster than your competition.
Institutional Intelligence: Every Study Makes You Smarter
The Hero’s Challenge: Each new program starts from scratch, losing institutional knowledge and repeating expensive mistakes.
The Guide’s Solution: RAG transforms every completed study into accessible intelligence for future programs.
Business Impact:
- Protocol design that leverages successful patterns from previous studies
- Patient stratification based on responder profiles across your portfolio
- Operational optimization using learnings from every trial you’ve ever run
- Competitive advantage that compounds with each new data point
Bottom Line: Your competitors run individual studies. You run an intelligence operation that gets smarter with every trial.
The Strategic Reality: While competitors struggle with fragmented insights and slow decision cycles, RAG-enabled sponsors cross from discovery to market authorization with the confidence that comes from unified intelligence, accelerated decisions, and risk-proofed innovation.
The Bottom Line
AI transformation in BioPharma isn’t about adopting the latest language model. It’s about building the bridge between AI potential and clinical reality.
We’re not Simon and Garfunkel, and this isn’t a song. But just like every hit needs a bridge to resolve the musical tension, BioPharma needs a platform like Xbiom whose RAG-enabled LLM model resolves the data tension between AI promises and clinical reality.
Ready to bridge the gap between AI dreams and BioPharma reality? The conversation starts with a simple question about your own data and ends with insights you never knew you had. Reach out to [email protected] for more info.

Andrew Parry is Director of Client Relations at PointCross Life Sciences, where he leverages his patient facing clinical experience and background in marketing and business development to help BioPharma companies optimize their data management strategies. Andrew specializes in building strategic partnerships that drive innovation and operational efficiency throughout the drug development lifecycle, with a passion for delivering life altering therapies to patient communities faster.

