The biopharmaceutical industry is entering a new era where the ability to harness data directly determines the speed and success of drug development. Clinical trials, nonclinical studies, and biomarker assays now generate massive volumes of complex data.
When unified and made accessible, clinical trial data accelerates insights, strengthens regulatory confidence, and reduces development risk.
PointCross enables this transformation through Xbiom, an enterprise platform that continuously harmonizes diverse data sources and embeds Retrieval-Augmented Generation (RAG) with Large Language Models (LLMs) inside secure firewalls. All study, trial, and assay data flows into a governed, scalable repository accessible through interoperable applications with strict role-based controls.
With a RAG-enabled chat interface, users across various functions, from monitors to scientists, can ask natural-language questions and receive validated, evidence-linked answers. Each response comes “with receipts,” anchored to source data and metadata, ensuring explainability and compliance while minimizing the risks of LLM hallucinations.
By combining the ease of LLMs with the rigor of RAG and the governance of Xbiom, PointCross turns fragmented ecosystems into unified, regulator-ready environments.
The result: faster cycle times, higher data confidence, and greater capacity for innovation across discovery, development, and submission.
The Current Challenge in BioPharma Data Ecosystems
Data Deluge Without Insight
Expanding Deliverables, Rising Demands: Regulatory submissions now encompass SDTM, ADaM, TFLs, CSRs, and emerging data types, including biomarkers and real-world evidence. These datasets must also be accessible to monitors and scientists. Without integration, opportunities for efficiency and discovery are lost.
Turning Inefficiencies into Automation Manual curation across EDCs, CRAs, and siloed systems delays decisions and adds integrity risks. A unified repository with interoperable applications and governed metadata (MDR) eliminates these bottlenecks, enabling automation, faster cycles, and more reliable insights.
Platforms Built for Today’s Scale Modern development pipelines demand platforms equal to the scale of the problem. Xbiom spans the full lifecycle, curation, smart transformation, search, analysis preparation, and visualization. With RAG-enabled AI, users interact with governed data directly in natural language, combining accessibility with the rigor of provenance-linked evidence.
Barriers to Enterprise AI Adoption
Safeguarding Data Sovereignty and Compliance. Strict residency and audit requirements prohibit proprietary trial data from leaving enterprise firewalls. Public LLMs (ChatGPT, Claude, Gemini) introduce risks by transmitting data externally without enforceable guarantees. Xbiom solves this with secure, in-firewall deployment aligned with FDA 21 CFR Part 11, EMA GCP, and ICH E6.
Simplifying Complex Infrastructures. Legacy environments, document management systems, data lakes, and statistical engines create friction that slows adoption. By unifying data into a governed repository, Xbiom turns complexity into an opportunity: a single, interoperable foundation for analytics and regulatory workflows.
Driving Adoption with Usability and Governance. High adoption barriers persist when end users face steep learning curves and executives lack direct access to explainable insights. Xbiom lowers these barriers by combining natural-language interaction with role-based governance. The result is democratized access: analysts, scientists, and leaders alike can explore data with confidence that every answer is explainable, validated, and regulatory-ready.
Takeaway
The future of BioPharma depends not on generating more data, but on converting it into trusted, actionable insight. Xbiom enables this transformation by unifying clinical, nonclinical, and biomarker data into a governed repository where information is interoperable, auditable, and ready for real-time use. With compliance-first controls, role-based governance, and a RAG-enabled interface, Xbiom ensures every stakeholder, from bench scientist to executive, can interact with data naturally, with provenance and regulatory confidence built in.
The result is a shift from bottlenecks and fragmentation to speed, clarity, and innovation.
The PointCross Solution: Xbiom Unified Repository
Xbiom was designed as an active data substrate, not another application layer, so we help pharma organizations fully leverage their data. It continuously harmonizes, validates, and contextualizes structured and unstructured sources into a single governed repository.
The platform accelerates discovery, strengthens compliance, and empowers every role in the enterprise.
Data Confidence and Provenance
Automated reconciliation supports accuracy and auditability, while regulators and scientists can drill down from summaries to raw evidence for complete transparency.
Speed with Rigor
Insights that once took weeks are delivered in hours. Harmonized ingestion accelerates decision-making without compromising statistical or regulatory integrity.
Conversational Access with Assurance
Through a RAG-enabled interface, users ask questions in plain language and receive answers with provenance, ensuring accessibility and trust while minimizing hallucinations.
Bridging Clinical and Nonclinical Domains
By connecting human and animal data, Xbiom enables safety signals, biomarker correlations, and adverse events to be analyzed across domains, supporting earlier risk detection and stronger translational decisions.
Enhanced Quality and Early Detection
Xbiom’s unified view enables pattern recognition missed in siloed approaches. For example, surfacing high-dose mortality patterns that had not been highlighted in official study outputs.
Next-Generation Modeling
Advanced ML pipelines extend analysis beyond traditional statistics, enabling continuous dose-response modeling and predictive safety signal detection that strengthen early discovery and de-risk development.
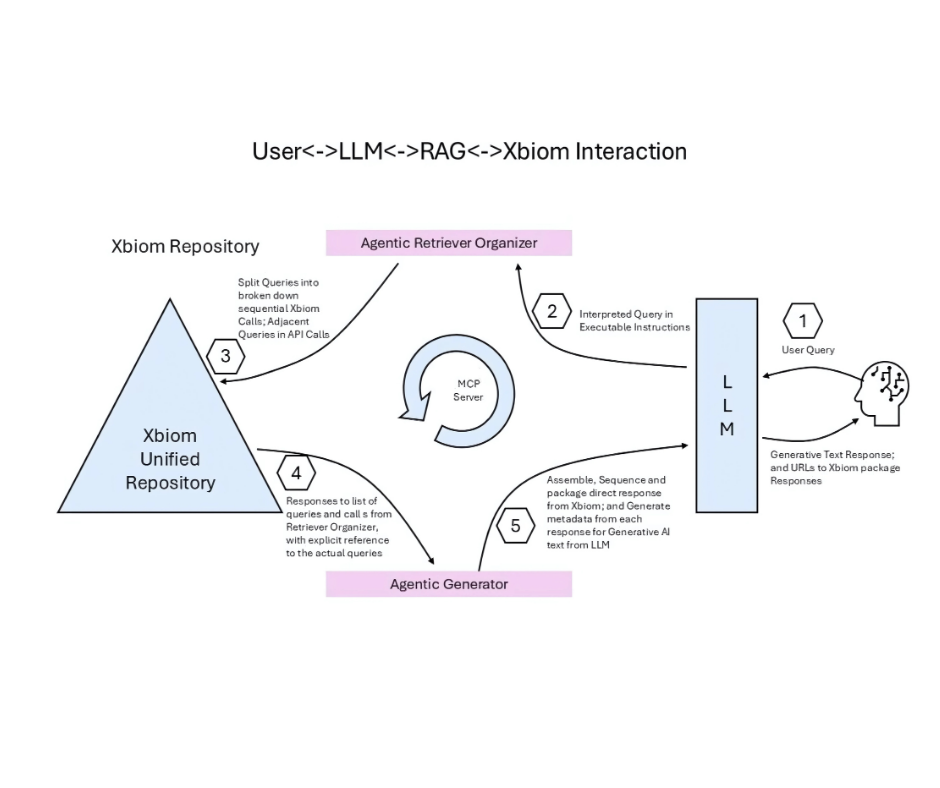
RAG + LLM + Xbiom: AI You Can Trust
- LLM Interpreter – Converts natural language into executable queries.
- RAG Retriever – Anchors answers in managed repositories, reducing hallucinations.
- Xbiom Repository – Maintains semantic consistency, metadata governance, and computational rigor.
LLM Interpreter, RAG Retriever and Xbiom Repository create explainable AI that delivers insights without compromising compliance.
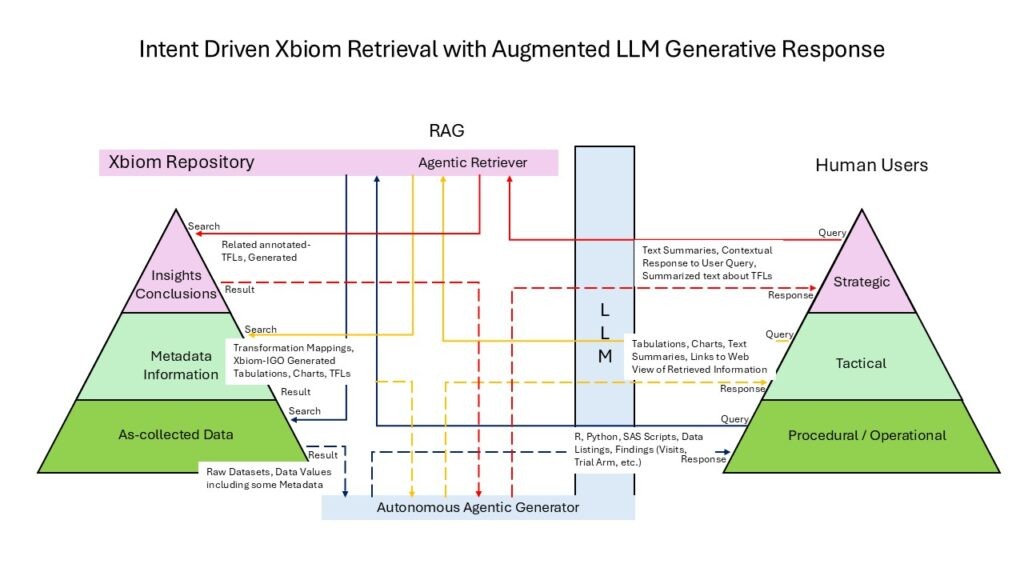
Role-Aware Design
- Operational (Data Layer): Analysts gain automation for standardization and validation.
- Tactical (Information Layer): Study leads and statisticians receive trusted cross-study outputs.
- Strategic (Knowledge Layer): Executives and regulators access audit-ready insights directly.
This role-aware framework closes the “translation gap” between those preparing the data and those making strategic decisions.
Security-First Deployment
Deployed behind enterprise firewalls, Xbiom uses biomedically trained, small-footprint LLMs. This ensures:
- Compliance with HIPAA, 21 CFR Part 11, SOC 2, and ISO 27001.
- Transparent provenance for every AI-assisted output.
- Governance that evolves as new modalities emerge.
- Interfaces tailored to users from bench scientist to C-suite.
Real-World Business Outcomes
- Accelerated cycles – Insights delivered in hours, not weeks.
- Reduced risk – Automated reconciliation minimizes resubmissions and delays.
- Faster discovery – Scientists test hypotheses and adapt trial design more rapidly.
- Empowered leadership – Executives engage directly with governed data.
Strategic Implications for the Industry
LLMs in BioPharma succeed only when embedded within governed repositories, not as standalone apps. Xbiom proves this model, demonstrating that only explainable, compliant, and interoperable AI can deliver measurable outcomes:
- Higher submission success rates.
- Earlier and more accurate safety signal detection.
- Faster therapeutic innovation.
Xbiom’s Three-Layer Architecture organizes content across three maturity levels that align with different user inquiry types
Conclusion
PointCross is redefining how humans interact with data in life sciences. By embedding RAG-enabled LLMs within Xbiom’s secure, governed architecture, organizations gain:
- Explainable AI with every answer linked to source evidence.
- Trusted governance ensuring metadata consistency across studies.
- Democratized access so every role, from analyst to executive, can interact with data confidently.
Vision: Asking questions about drug development data should be as simple as having a conversation. With Xbiom, that vision is already being realized today.

About the Author: Aruna Adhikari Thapa is the Chief Product and Commercial Officer at PointCross Life Sciences, and a 2024 PharmaVoice 100 honoree recognized for her leadership in advancing the use of technology and AI across clinical development and regulatory operations. She is a product and commercial expert with over 20 years of experience scaling AI-powered SaaS businesses, transforming healthcare through data innovation, and driving enterprise growth across regulated industries. Aruna Adhikari Thapa, MBA, Chief Product and Commercial Officer.

